Figure 5.
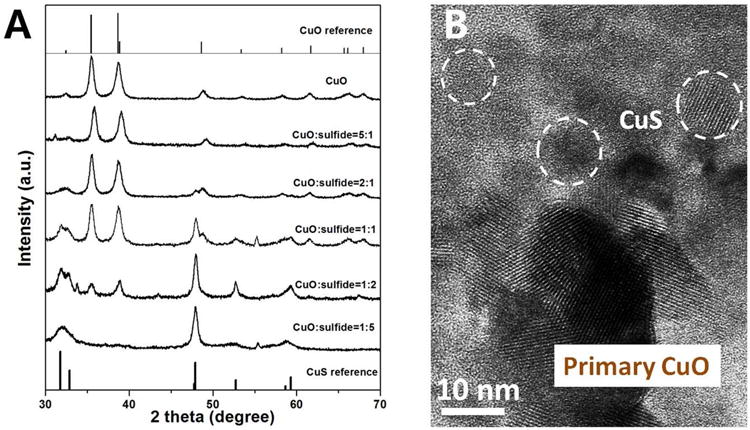
Phases and morphologies of the solid products from reaction of CuO NPs with soluble HS-. (A) XRD patterns of sulfidated CuO nanoparticles generated from initial Cu/S ratios that vary from 0.2 to 5. CuO (tenorite) and CuS (covellite) reference is presented for comparison. Please note the CuO/CuS peak intensity ratio can't be used to calculate the percentage of CuS produced, but it can show a general trend that CuS produced increase with decreasing CuO to bisulfide ratio. (B) HRTEM image of sulfidated CuO nanoparticles (generated from 2.5 mM of CuO NPs incubated in 5mM Na2S solution for one day), corresponding to a CuO/bisulfide molar ratio of 1:2. The images show the formation of small secondary particles with size 5 to 10 nm, and showing lattice fringes indicating the presence of at least some crystalline products.
