Figure 7.
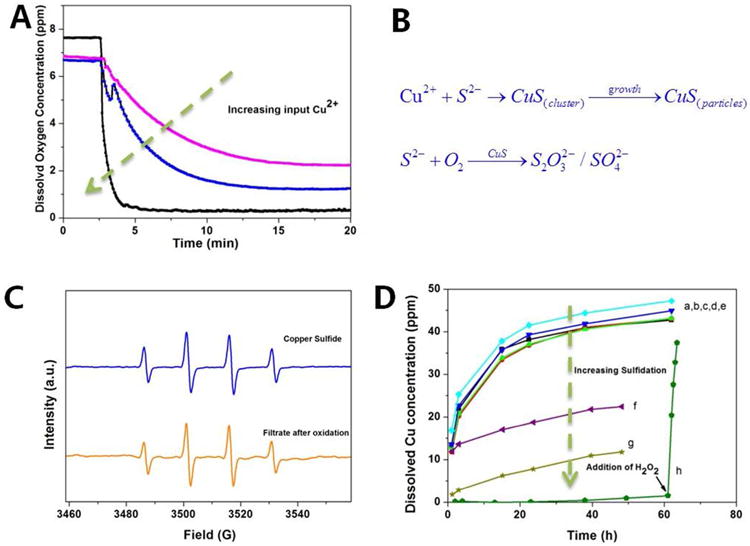
Properties of sulfidated CuO NPs and implications for toxicity. (A,B) CuS clusters and nanoparticles serve as catalysts for bisulfide oxidation. Reaction tracked through depletion of dissolved oxygen upon addition of copper ions to excess Na2S solution. Pink, blue and black curves correspond to addition of 0.1, 1 or 2 mM copper ion. (C) Hydroxyl radical EPR signal induced by a 0.1 mM CuS suspension and its particle-free filtrate after hydrogen peroxide treatment. Despite low solubility, CuS shows redox activity associated with soluble species. (D) Dissolution behavior of CuS and a series of partially sulfidated CuO NPs at pH 4 acetate buffer. The sulfidation was carried out using 2.5mM CuO at the initial S/Cu ratios from 0.2 to 5. (a, b, c, d, e represents the dissolution of CuO, sulfidated CuO with S/Cu at 0.1, 0.2,0.5,1, which has little effect on CuO dissolution; f, g represents the dissolution behavior of sulfidated CuO with S/Cu at 2,5 respectively, which can slow the dissolution rate.) The pure CuS solid was prepared through precipitation of Cu2+ and bisulfide in stoichiometric mixture followed by washing and resuspension. To understand the redox behavior in (C) the oxidative-dissolution of CuS was investigated by adding H2O2 solution in equimolar proportion to CuS.
