Abstract
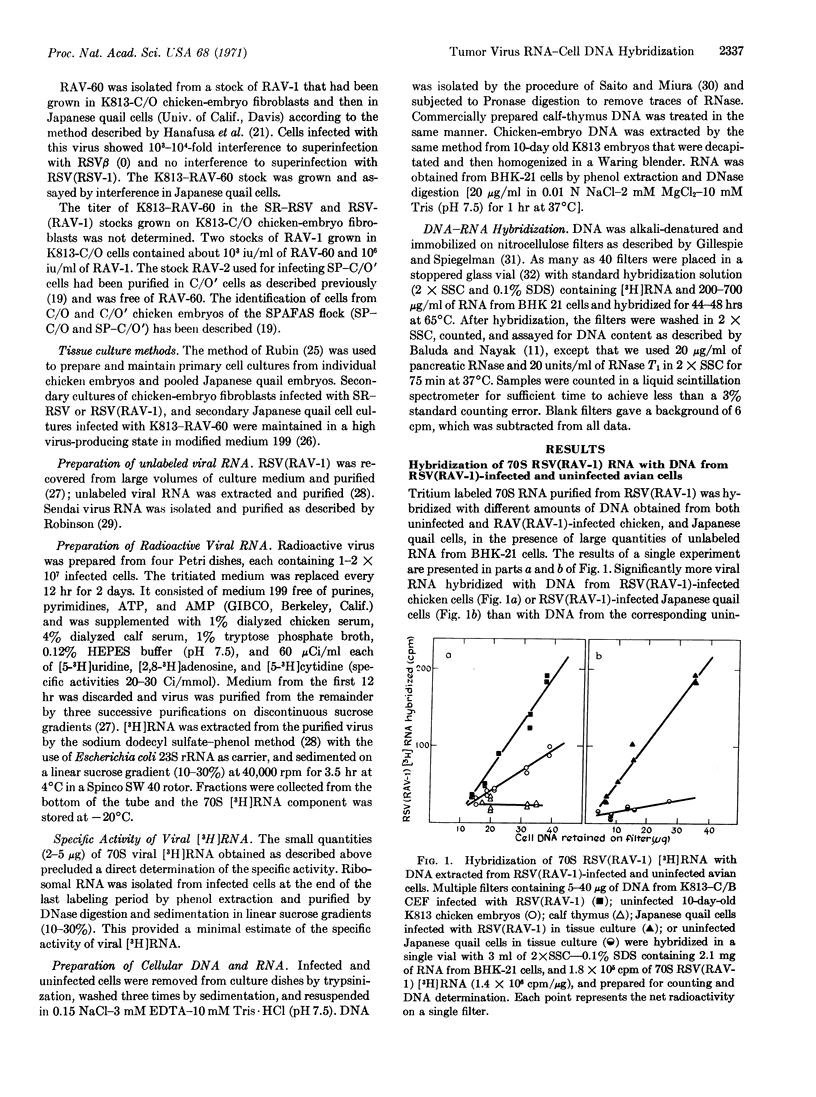
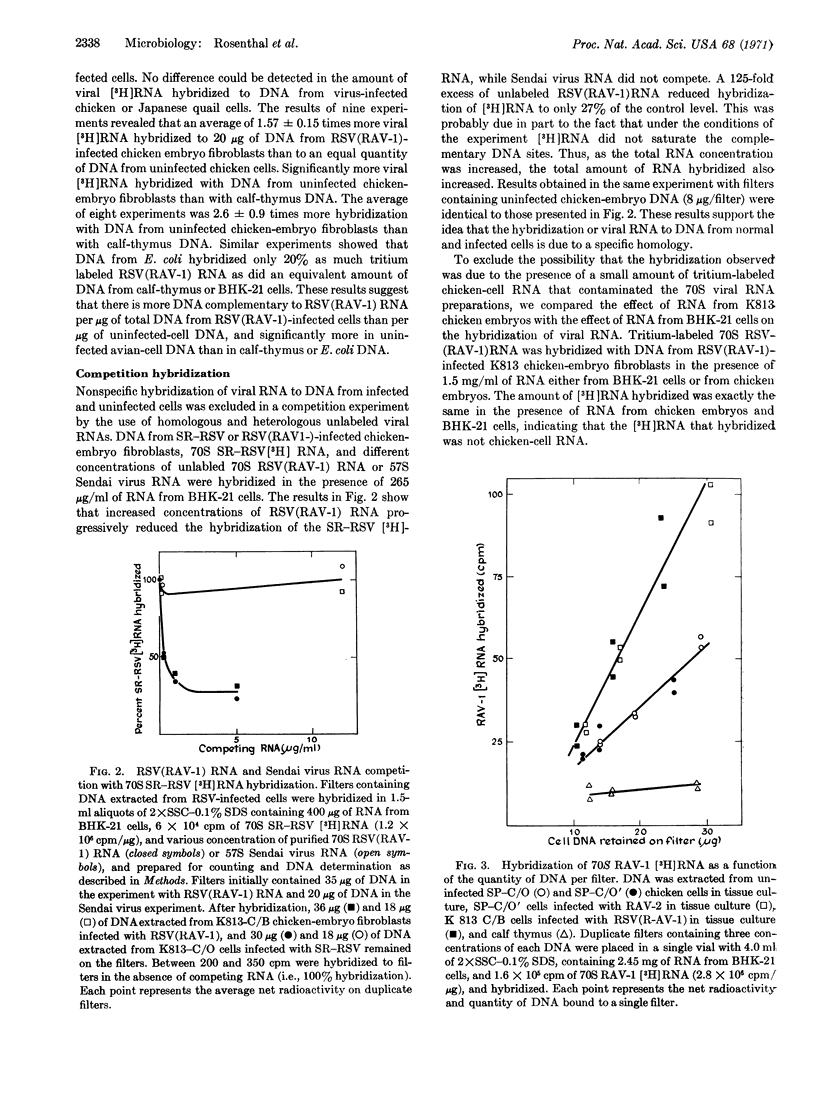
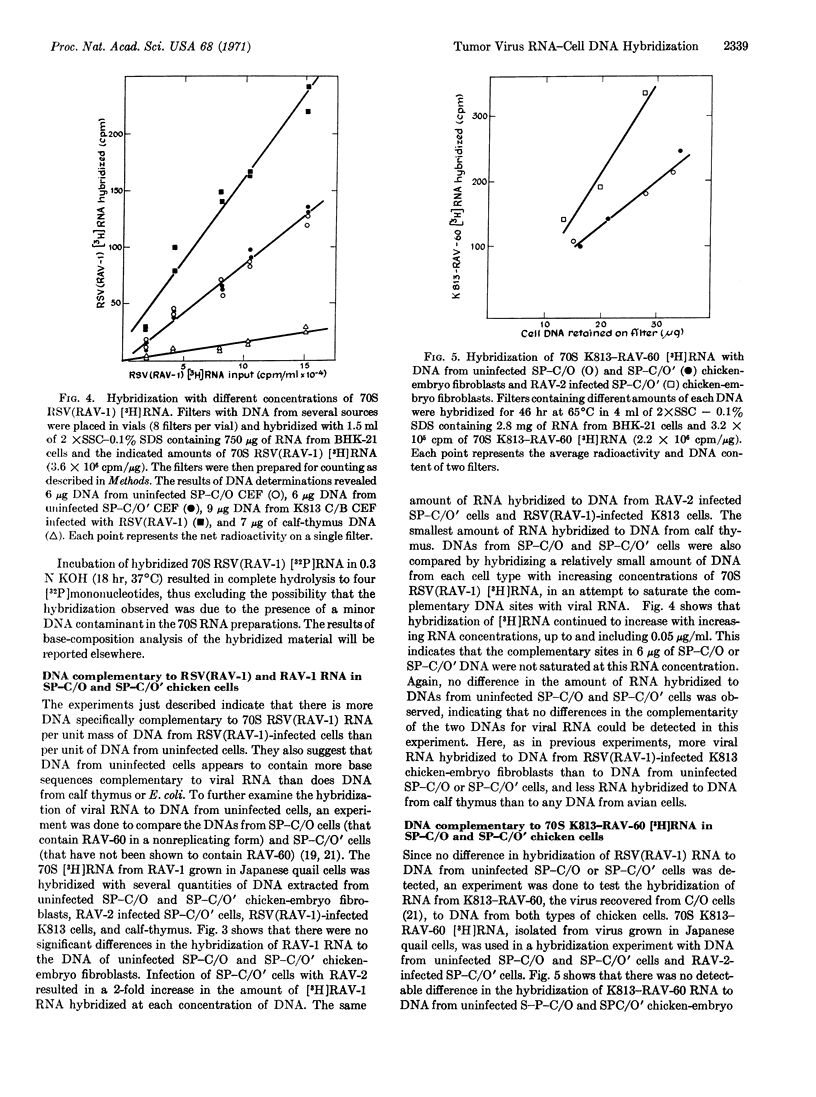
The 70S RNA component of several avian tumor viruses was hybridized with DNA extracted from avian tumor virus-infected and uninfected chicken and Japanese quail cells. Tritium-labeled 70S RNAs from Rous sarcoma virus (RSV), Rous associated virus-1 (RAV-1), RAV-60, and Schmidt-Ruppin-RSV (SR-RSV) hybridize from 3 to 10 times more with DNA from uninfected chicken cells than with DNA from Escherichia coli, calfthymus, or baby hamster kidney cells. After infection of chicken cells with RSV(RAV-1), SR-RSV, or RAV-2, the amount of 70S avian tumor virus [3H]RNA hybridized increases by 1.6 times. The specificity of the hybridization reaction was shown by the specific competition of 70S SR-RSV [3H]RNA with 70S RNA from RSV(RAV-1), and not with RNA from Sendai virus or chicken cells. There was no difference in the hybridization of 70S RNA from RSV (RAV-1), RAV-1, or RAV-60 with DNA either from chicken cells that contain RAV-60 in a nonreplicating form or from chicken cells that do not appear to contain RAV-60. These results indicate that both types of uninfected chicken cells contain DNA that is complementary to RNA from several avian tumor viruses and that the amount of complementary DNA increases in such cells after infection with an avian tumor virus. The RNAs of genetically different avian tumor viruses appear to have indistinguishable base sequences by this technique.
Keywords: Rous sarcoma virus, Rous associated virus, RNA-DNA hybrids
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BADER J. P. THE REQUIREMENT FOR DNA SYNTHESIS IN THE GROWTH OF ROUS SARCOMA AND ROUS-ASSOCIATED VIRUSES. Virology. 1965 Jun;26:253–261. doi: 10.1016/0042-6822(65)90272-2. [DOI] [PubMed] [Google Scholar]
- BADER J. P. THE ROLE OF DEOXYRIBONUCLEIC ACID IN THE SYNTHESIS OF ROUS SARCOMA VIRUS. Virology. 1964 Apr;22:462–468. doi: 10.1016/0042-6822(64)90067-4. [DOI] [PubMed] [Google Scholar]
- Bader J. P. Metabolic requirements for infection by Rous sarcoma virus. I. The transient requirement for DNA synthesis. Virology. 1966 Jul;29(3):444–451. doi: 10.1016/0042-6822(66)90220-0. [DOI] [PubMed] [Google Scholar]
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970 Jun 27;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Baluda M. A., Nayak D. P. DNA complementary to viral RNA in leukemic cells induced by avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1970 Jun;66(2):329–336. doi: 10.1073/pnas.66.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. D., Weber C. S. Gene linkage by RNA-DNA hybridization. I. Unique DNA sequences homologous to 4 s RNA, 5 s RNA and ribosomal RNA. J Mol Biol. 1968 Jun 28;34(3):661–680. doi: 10.1016/0022-2836(68)90188-5. [DOI] [PubMed] [Google Scholar]
- Dougherty R. M., Di Stefano H. S. Lack of relationship between infection with avian leukosis virus and the presence of COFAL antigen in chick embryos. Virology. 1966 Aug;29(4):586–595. doi: 10.1016/0042-6822(66)90282-0. [DOI] [PubMed] [Google Scholar]
- Duff R. G., Vogt P. K. Characteristics of two new avian tumor virus subgroups. Virology. 1969 Sep;39(1):18–30. doi: 10.1016/0042-6822(69)90344-4. [DOI] [PubMed] [Google Scholar]
- Hanafusa H., Hanafusa T. Determining factor in the capacity of Rous sarcoma virus to induce tumors in mammals. Proc Natl Acad Sci U S A. 1966 Mar;55(3):532–538. doi: 10.1073/pnas.55.3.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa H., Miyamoto T., Hanafusa T. A cell-associated factor essential for formation of an infectious form of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1970 Jun;66(2):314–321. doi: 10.1073/pnas.66.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa T., Hanafusa H., Miyamoto T. Recovery of a new virus from apparently normal chick cells by infection with avian tumor viruses. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1797–1803. doi: 10.1073/pnas.67.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa T., Miyamoto T., Hanafusa H. A type of chick embryo cell that fails to support formation of infectious RSV. Virology. 1970 Jan;40(1):55–64. doi: 10.1016/0042-6822(70)90378-8. [DOI] [PubMed] [Google Scholar]
- Hung P. P., Robinson H. L., Robinson W. S. Isolation and characterization of proteins from Rous sarcoma virus. Virology. 1971 Jan;43(1):251–266. doi: 10.1016/0042-6822(71)90243-1. [DOI] [PubMed] [Google Scholar]
- Payne L. N., Chubb R. C. Studies on the nature and genetic control of an antigen in normal chick embryos which reacts in the COFAL test. J Gen Virol. 1968 Dec;3(3):379–391. doi: 10.1099/0022-1317-3-3-379. [DOI] [PubMed] [Google Scholar]
- Robinson H. L., Robinson W. S. Inhibition of growth of uninfected and rous sarcoma virus-infected chick-embryo fibroblasts by rifampicin. J Natl Cancer Inst. 1971 Apr;46(4):785–788. [PubMed] [Google Scholar]
- Robinson W. S., Pitkanen A., Rubin H. The nucleic acid of the Bryan strain of Rous sarcoma virus: purification of the virus and isolation of the nucleic acid. Proc Natl Acad Sci U S A. 1965 Jul;54(1):137–144. doi: 10.1073/pnas.54.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson W. S. Self-annealing of subgroup 2 myxovirus RNAs. Nature. 1970 Mar 7;225(5236):944–945. doi: 10.1038/225944a0. [DOI] [PubMed] [Google Scholar]
- Rubin H. A VIRUS IN CHICK EMBRYOS WHICH INDUCES RESISTANCE IN VITRO TO INFECTION WITH ROUS SARCOMA VIRUS. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1105–1119. doi: 10.1073/pnas.46.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- TEMIN H. M. HOMOLOGY BETWEEN RNA FROM ROUS SARCOMA VIROUS AND DNA FROM ROUS SARCOMA VIRUS-INFECTED CELLS. Proc Natl Acad Sci U S A. 1964 Aug;52:323–329. doi: 10.1073/pnas.52.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEMIN H. M. THE EFFECTS OF ACTINOMYCIN D ON GROWTH OF ROUS SARCOMA VIRUS IN VITRO. Virology. 1963 Aug;20:577–582. doi: 10.1016/0042-6822(63)90282-4. [DOI] [PubMed] [Google Scholar]
- TEMIN H. M. THE PARTICIPATION OF DNA IN ROUS SARCOMA VIRUS PRODUCTION. Virology. 1964 Aug;23:486–494. doi: 10.1016/0042-6822(64)90232-6. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970 Jun 27;226(5252):1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]
- VIGIER P., GOLDE A. EFFECTS OF ACTINOMYCIN D AND OF MITOMYCIN C ON THE DEVELOPMENT OF ROUS SARCOMA VIRUS. Virology. 1964 Aug;23:511–519. doi: 10.1016/0042-6822(64)90235-1. [DOI] [PubMed] [Google Scholar]
- Vogt P. K., Ishizaki R. Reciprocal patterns of genetic resistance to avian tumor viruses in two lines of chickens. Virology. 1965 Aug;26(4):664–672. doi: 10.1016/0042-6822(65)90329-6. [DOI] [PubMed] [Google Scholar]
- Yoshikawa-Fukada M., Ebert J. D. Hybridization of RNA from Rous sarcoma virus with cellular and viral DNA's. Proc Natl Acad Sci U S A. 1969 Nov;64(3):870–877. doi: 10.1073/pnas.64.3.870. [DOI] [PMC free article] [PubMed] [Google Scholar]


