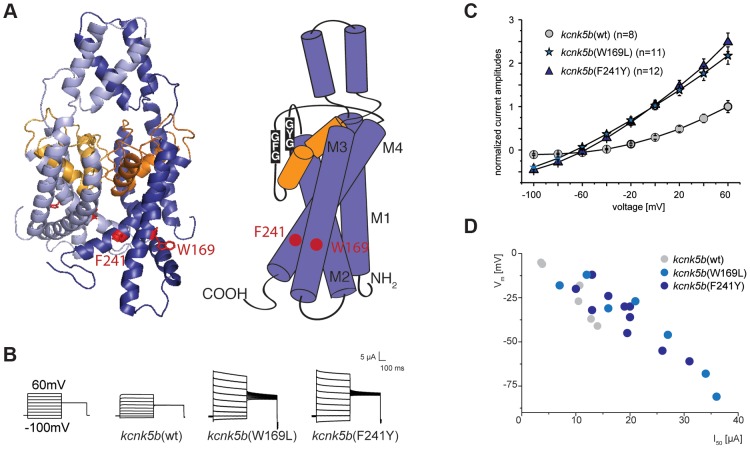
Figure 5. Gain-of-function mutations in kcnk5b affect ionic conduction and lead to hyperpolarization of the cell.
(A) Location of the amino acids altered in kcnk5b gain-of-function mutants. Kcnk5b protein was modeled on human KCNK4 (K2p4.1). GFG and GYG domains represent the selectivity pore of the channel. (B) Voltage clamp recordings from Xenopus oocytes injected with cRNA of wild type and mutant kcnk5b. The membrane potential was clamped at a reference potential of −80 mV and then stepped to a test potential from +60 mV to −100 mV for 500 ms. The current that is applied in order to clamp the voltage to a certain value corresponds to the current passing through the plasma membrane. Representative electrophysiological traces are shown. (C) The mutant channels display increased conductance over wild type channels expressed at comparable levels. Error bars represent standard deviation. (D) Kcnk5b influences membrane potential (Vm) in oocytes. The mutant variants tend to hyperpolarize the cell (each point represents one oocyte).