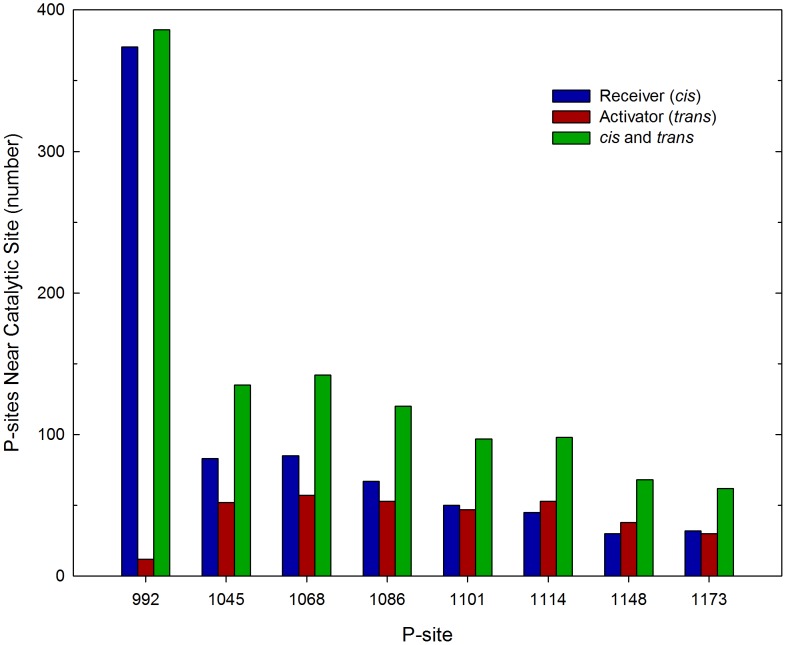
Figure 6. Number of P-sites in proximity of the catalytic site in randomized EGFR structural models.
The set of five hundred dimeric EGFR structures with randomized CT domain conformations was analyzed to determine the number of structures that had a given P-site tyrosine residue within 40 Å of the γ-phosphate of the AMPPMP substrate bound in the catalytic site. The kinase-proximal site P-site-992 of the receiver molecule was most often and that of the activator molecule least often near the catalytic site, consistent with the bias for cis versus trans P-site-992 binding events in simulations (cf. Fig. 4). This cis-trans bias, as well as the number in proximity of the catalytic site, was markedly reduced for P-sites more distal to the kinase core.