Figure 4. Electron density maps for inhibitors bound to SHV-1 β-lactamase.
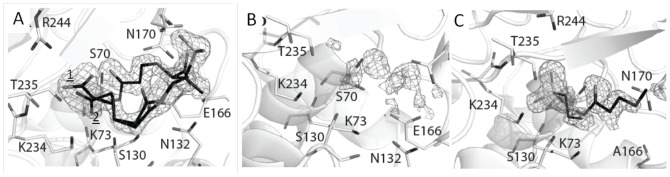
(A) |Fo|-|Fc| electron density difference map contoured at 3.0σ for PSR-4-157 prior to inclusion in refinement. The C2 carboxyl-tail linker was observed to be in two conformations. (B) |Fo|-|Fc| difference density for PSR-4-155 soaked wt SHV-1 soaked crystals revealing an absence of strong, interpretable inhibitor density. (C) |Fo|-|Fc| electron density difference map contoured at 3.0σ for PSR-3-226 prior to inclusion in refinement in the E166A SHV structure. Observed density reveals presence of a partially ordered PSR-3-226 fragment, right, and a HEPES buffer molecule fragment, left.
