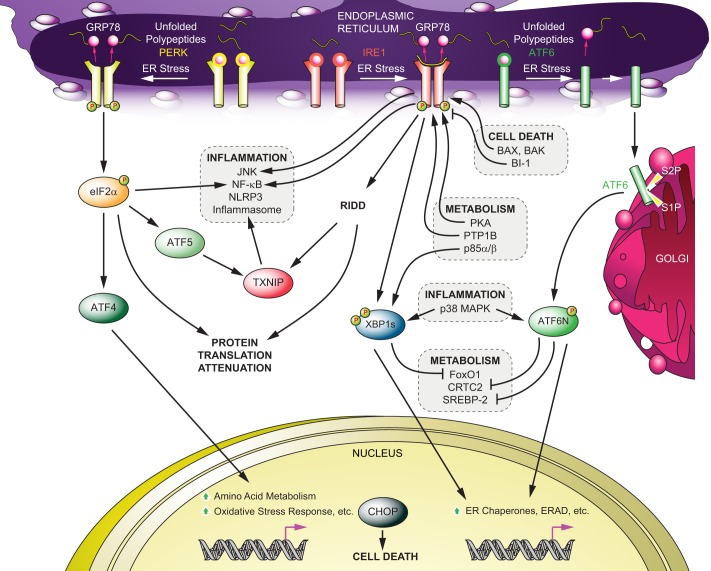
FIGURE 1.
UPR signaling and its cross-talk mediated by IRE1-XBP1, PERK and ATF6. Upon ER stress, the dissociation of GRP78 from the lumenal domain of IRE1, PERK, and ATF6 initiates UPR signaling. The direct binding of unfolded proteins to IRE1 also activates IRE1-mediated UPR. Active IRE1 selectively splices XBP1 mRNA to produce an active transcription factor (XBP1s) or down-regulates mRNAs or microRNAs (RIDD). Activated PERK phosphorylates eIF2α to attenuate global protein translation while selectively augmenting the translation of ATF4 and ATF5. An active form of ATF6 is generated upon cleavage by S1P and S2P after translocation to the Golgi from the ER. There are different cross-talks between UPR signaling components and other signal transduction pathways, such as inflammatory pathways (JNK, NF-κB, p38MAPK, and the NLRP3 inflammasome), apoptotic machinery (Bax, Bak, and BI-1), and metabolism (p85α/β, FoxO1, CRTC2, and SREBP-2). ERAD, ER-associated degradation.