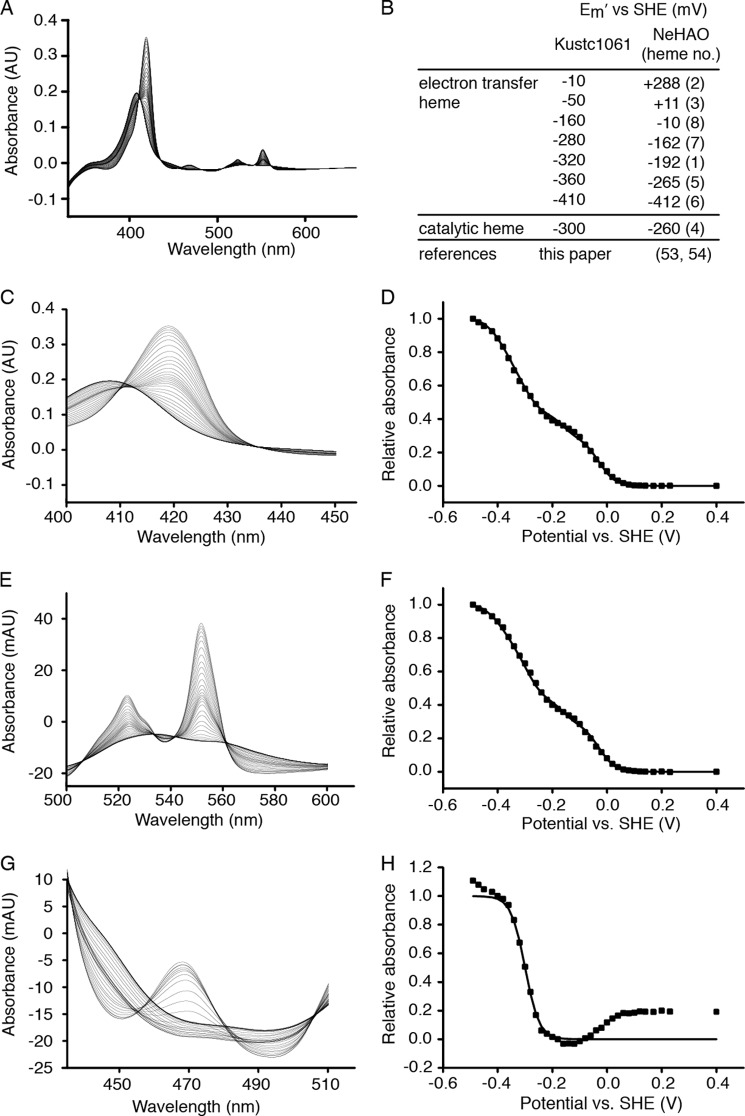
FIGURE 3.
Protein film electrochemistry of kustc1061. A, full spectra of the oxidative titration from −490 to +230 mV versus standard hydrogen electrode (SHE) in 20-mV steps. B, table showing the Em′ values (± 10 mV) calculated for the successive oxidations and reductions of the c-type hemes in kustc1061 and the comparison to those reported for NeHAO (53, 54). Numbers in parentheses refer to the corresponding hemes in NeHAO. Note that the heme c midpoint redox potentials in kustc1061 are generally lower, but the method applied does not allow the assignment to specific hemes, except for the catalytic heme 4. C, spectral details of the Soret region. D, signals at 418 nm normalized to 1 and plotted against the applied potentials. E, spectral details of the heme c-α and -β bands. F, signals at 551 nm normalized to 1 and plotted against the applied potentials. G, spectral details of the P460 region. H, signals at 468 nm normalized to 1 and plotted against the applied potentials. Squares in D, F, and H represent experimental values. Solid lines in these graphs are Nernstian fits. The data in D and F were well described by the sum of seven Nernstian contributions from isolated, single-electron (n = 1) redox centers having the Em′ values listed in B (all ± 10 mV). It was assumed that P460 spectral properties did not interfere significantly with the spectral changes of the Soret (C) and α and β (E) bands of the His/His ligated hemes; clear isosbestic points at 435 nm and at 505 and 561 nm, respectively, supported this assumption. The variation in absorbance at 468 nm had contributions from redox transformation of the P460 co-factor in addition to those of low spin His/His ligated hemes. The changes in absorbance caused by the P460 co-factor were readily identified through their larger magnitude and the appearance of a peak at low potential with an isosbestic point at 455 nm. The plot of normalized A468 nm − A455 nm was well described by a fit to the Nernst equation for an n = 1 center with Em′ = −300 mV.