FIGURE 4.
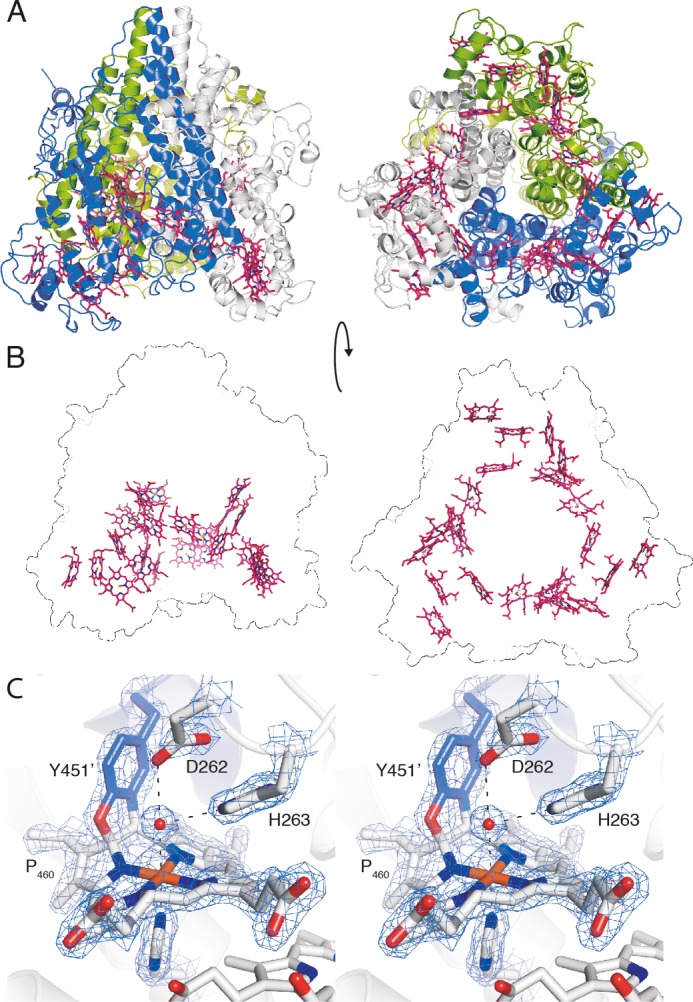
X-ray crystal structure of kustc1061 from K. stuttgartiensis. A, views of the kustc1061 trimer perpendicular to (left panel) and along the 3-fold symmetry axis (right panel). The three monomers are shown in different colors. The 24 heme groups are shown in red. B, the same views of the kustc1061 trimers, showing the outline of the protein and the ring-like arrangement of heme groups (red). C, stereo figure, showing a close-up of the active site of kustc1061. The P460 co-factor consists of a heme c moiety (white sticks) covalently bound in two places to the Tyr451 side chain of an adjacent protein monomer (blue). The conserved Asp262/His263 is shown, as well as His227, which coordinates the heme iron as the proximal ligand. A water molecule (red sphere) forms the distal ligand. The final, refined 2mFo − DFc electron density map calculated from 1.8 Å resolution data is overlaid at a 2 σ contour level (blue mesh). The figures were prepared in PyMOL (47).
