FIGURE 2.
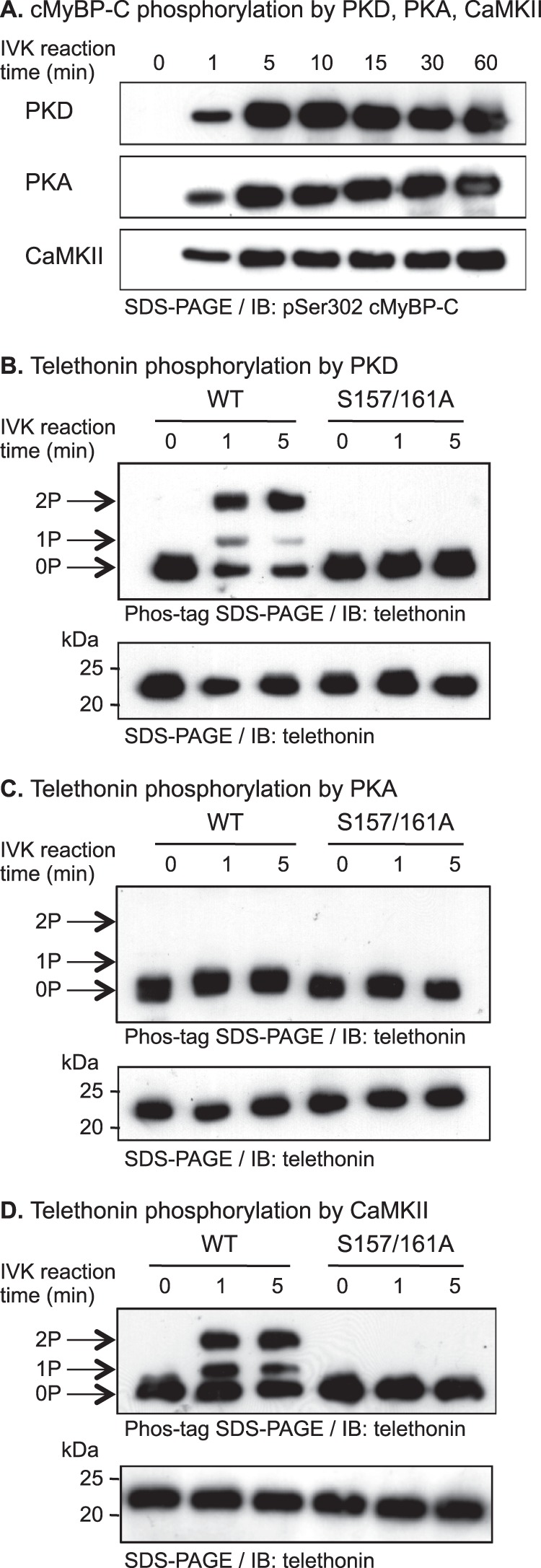
Telethonin is phosphorylated at Ser-157 and Ser-161 by PKD and CaMKII but not by PKA. A, comparable phosphorylation by PKD, PKA, or CaMKII of Ser-302 in the recombinant cardiac myosin-binding protein C (cMyBP-C) c1c2 fragment in vitro. A recombinant His6-tagged c1c2 fragment (100 pmol) was incubated in the presence of each kinase for 0–60 min and subjected to immunoblot (IB) analysis using a phospho-specific pSer-302 cMyBP-C antibody. IVK, in vitro kinase. B–D, differential phosphorylation by PKD (B), PKA (C), or CaMKII (D) of recombinant telethonin in vitro. Recombinant His6-tagged WT or S157A/S161A telethonin proteins (100 pmol) were incubated in the absence or presence of each kinase for 0, 1, or 5 min and subjected to Phos-tag SDS-PAGE and immunoblot analysis using a monoclonal anti-telethonin antibody (top panel). Arrows on the left show the identities of dual-phosphorylated (2P), mono-phosphorylated (1P), and non-phosphorylated (0P) telethonin. The bottom panel shows the same samples subjected to standard SDS-PAGE and immunoblot analysis using the same antibody.
