FIGURE 5.
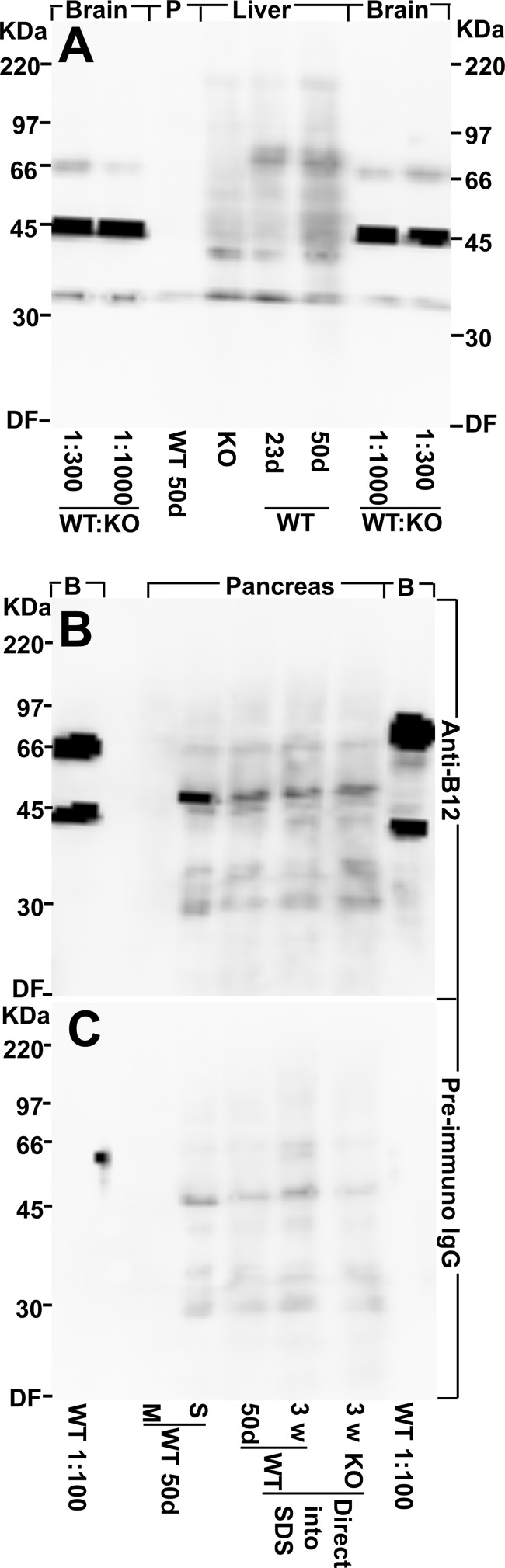
Western blots show that there is very little, if any, EAAT2 protein in pancreas. A, EAAT2 protein is detectable by immunoblotting in brain and liver but not in pancreas (P). Various tissues from a 50-day-old (50d) male WT mouse and from 23-day-old (23d) conventional EAAT2 KO and WT littermates were collected, homogenized in water with protease inhibitors (see “Experimental Procedures”), and centrifuged. The pellets (the water-insoluble fraction containing cell membranes) were extracted with SDS. The WT forebrain extract (50 days old) was diluted 300 or 1,000 times as indicated in the KO forebrain extract. Note that EAAT2 is detectable in the diluted WT brain extracts and in the WT liver extracts (both 23 and 50 days old) but not in the extracts from pancreas (50 days old) or in the KO liver extract. The band representing EAAT2 is the one just above the 66-kDa marker, whereas the strong 45-kDa band seen in the brain samples is due to a cross-reacting compound. The latter is strong relative to EAAT2 because it is present in both the WT and the KO extract and thereby not diluted like EAAT2. The total protein loaded in each lane was 30 μg. The blot was developed with EAAT2 anti-B12 antibodies (Ab360; 0.2 μg/ml). B, pancreas contains water-soluble proteins that bind the antibodies, and these unidentified proteins have a molecular mass similar to that of EAAT2. Pancreas from a 50-day-old WT mouse was homogenized in water with protease inhibitors (see “Experimental Procedures”) and centrifuged to separate the water-soluble (S) proteins from the membrane fraction (M). Note that there is no labeling in the lane containing the pancreas membrane fraction. All of the labeling is due to the water-soluble components. In agreement, there is similar labeling when whole pancreas (50-day-old WT, 23-day-old WT, and 23-day-old KO) were directly solubilized in SDS. The membrane fraction from WT forebrain (50 days old) diluted 1:100 with KO forebrain extract (B) was used as a positive control (the lanes on each side of the blot as indicated). Note that the 66-kDa band representing EAAT2 is stronger relative to the cross-reacting 45-kDa band than in A due to less dilution. The blot was developed with EAAT2 anti-B12 antibodies (Ab360; 0.2 μg/ml). C, a blot identical to that in B was developed with normal (preimmune) IgG (0.2 μg/ml). Note that the extracts from whole pancreas and from the water-soluble fraction contain proteins that tend to bind IgG in general. In particular, there is weak labeling of a band that can easily be mistaken for EAAT2 when high sensitivity detection systems are used. DF, dye front.