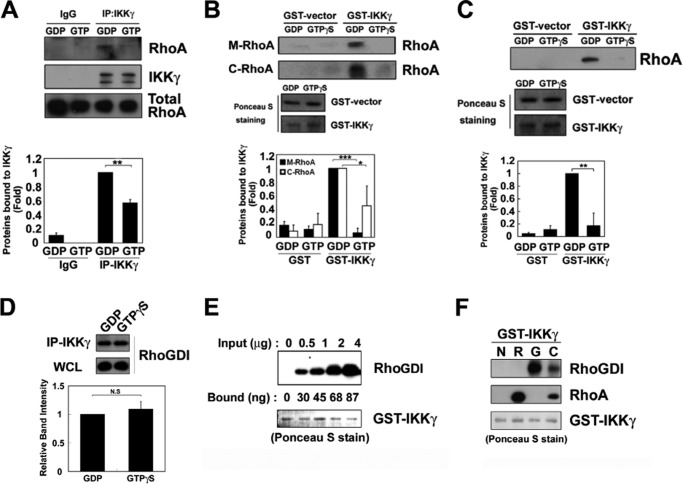
FIGURE 2.
RhoA and RhoGDI interact with IKKγ. A, RAW264.7 cell lysates were preloaded with 0.1 mm GTPγS or 1 mm GDP for 30 min. The lysates were subjected to immunoprecipitation with an anti-IKKγ antibody and analyzed by Western blot with the anti-RhoA and anti-IKKγ antibodies. B, RhoA proteins purified from the membrane (M-RhoA) and cytosolic fractions (C-RhoA) of Sf9 cells, preloaded with 1 mm GDP or 0.1 mm GTPγS, were precipitated with purified GST-IKKγ-Sepharose 4B beads. The GST and GST-IKKγ levels were measured with Ponceau S staining. C, recombinant RhoA protein (1 μg) expressed in E. coli was preloaded with 1 mm GDP or 0.1 mm GTPγS and then precipitated with purified GST-IKKγ-Sepharose 4B beads. GST and GST-IKKγ were identified with Ponceau S staining. The data represent the means ± S.E. of three independent experiments (*, p < 0.05; **, p < 0.01; ***, p < 0.001). D, lysates of RAW264.7 cells (500 μg of protein) were preloaded with 1 mm GDP and 0.1 mm GTPγS for 30 min at 30 °C. WCL, whole cell lysate. IKKγ was immunoprecipitated using 2 μg of anti-IKKγ antibody for 2 h at 4 °C. Co-precipitated RhoGDI was detected by Western blot analysis. E, 1 μg of GST-IKKγ conjugated to glutathione beads was incubated with 0.5–4 μg of recombinant RhoGDI for 2 h at 4 °C. RhoGDI bound to IKKγ was detected by Western blot. The amount of RhoGDI bound to IKKγ was calculated from the control standard curve. GST-IKKγ was detected with Ponceau S staining. F, 1 μg of GST-IKKγ conjugated to glutathione beads (GSH) was incubated with 2 μg of recombinant RhoA (R) purified from Sf9 cell membranes, 2 μg of purified RhoGDI (G), and 2 μg of purified RhoA-RhoGDI complex (C) for 2 h at 4 °C. RhoA and RhoGDI bound to IKKγ were detected by Western blot.