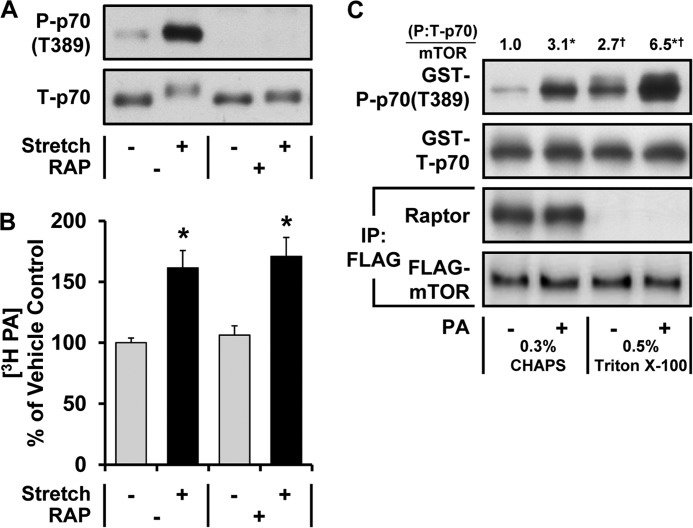
FIGURE 1.
Evidence that PA can function as a direct upstream activator of mTOR signaling in response to mechanical stimulation. A and B, mouse EDL muscles were held at Lo in an ex vivo organ culture system and treated as follows. A, preincubated with 150 nm rapamycin (RAP +) or the vehicle (RAP −, DMSO) for 30 min and then subjected to 90 min of a stretch (Stretch +) or control condition (Stretch −) followed by Western blot analysis for phosphorylated (P) and total (T) p70. B, prelabeled with [3H]myristic acid for 2 h. During the final 30 min of the prelabeling, the muscles were incubated with rapamycin or the vehicle as in A and then subjected to 90 min of the stretch or control conditions. The concentration of 3H-labeled PA was measured and expressed as a percentage of values obtained in the vehicle control samples. C, C2C12 myoblasts stably expressing FLAG-mTOR were serum-starved overnight and collected in either CHAPS or Triton X-100 lysis buffer. The lysates were subjected to immunoprecipitation (IP) for the FLAG epitope, and then the immunoprecipitates were incubated for 15 min with 150 μm PA vesicles (PA +) or 150 μm PC vesicles as a control condition (PA −). The kinase activity of mTOR was then assayed with GST-p70 as a substrate. The resulting samples were subjected to Western blot analysis for the indicated proteins, and the phosphorylated:total ratios of GST-p70 were divided by the amount of mTOR in each reaction. These values were then expressed as a ratio of the values obtained in the PC-treated samples collected in CHAPS lysis buffer. The values were obtained from four independent experiments. All values are presented as the mean (±S.E. in graphs, n = 3–11 per group). *, significantly different from the drug (B)- or lysis buffer (C)-matched control group. †, significantly different from the stimulation-matched CHAPS group; p ≤ 0.05.