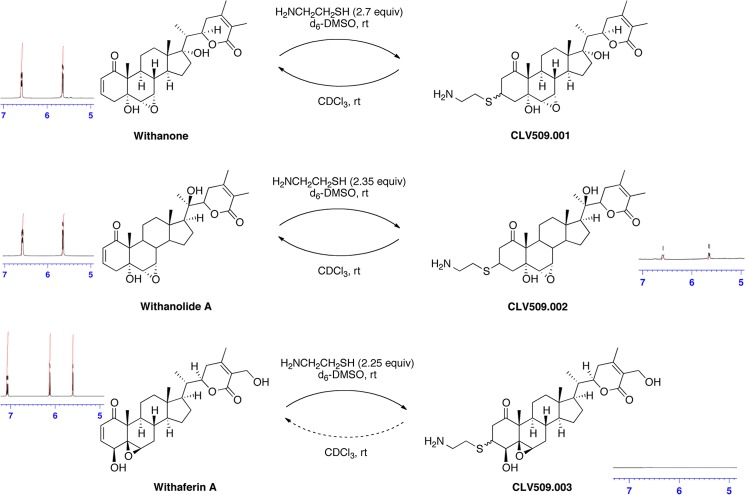
FIGURE 7.
Thiol reactivity of withanolides determined by NMR. Shown are the results of NMR trapping experiments with cysteamine as the nucleophile and WE, WLA, and WA as electrophiles. For WE, no difference was observed at the enone peaks at 6.58 and 5.64 ppm. For WLA, the enone peaks at 6.58 and 5.64 ppm decreased by 25% after 5 min of incubation, but these peaks were present in full intensity after 5 h. For WA, the enone peaks at 7.08 and 6.12 ppm disappeared 5 min after thiol addition and did not reappear. Thus, only WA resulted in an irreversible addition of the thiol to the enone moiety.