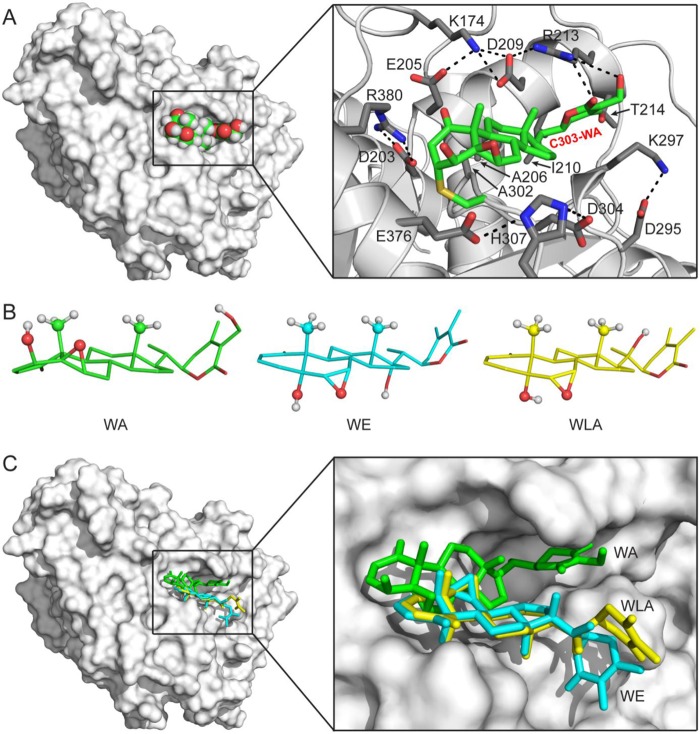
FIGURE 9.
Docking of the Cys303-WA adduct in human β-tubulin. A, model of the Cys303-WA adduct of human β-tubulin. Left, overall view of the model. The human β-tubulin model is shown as a molecule surface, and the Cys303-WA adduct is shown as a sphere model with an atomic color scheme (carbon in green, oxygen in red, and hydrogen in white). Right, details of the WA-binding pocket. The human β-tubulin model is shown as a ribbon diagram (helices as spirals, strands as arrows, and loops as tubes) with WA-interacting side chains as sticks (nitrogen in blue, carbon in gray, and oxygen in red), and the Cys303-WA adduct is shown as a stick model (nitrogen in blue, carbon in green, oxygen in red, and sulfur in yellow). Hydrogen atoms are not shown for clarity. B, comparison of the WA, WE, and WLA structures (PubChem Database SID 11034, 103600366, and 103600367, respectively). The three structures are aligned on the basis of their fused rings. Free rotations around side chain single bonds are allowed. For clarity, hydrogen atoms are shown for the hydroxyl and axial methyl groups only. C, WE and WLA are not compatible for Cys303 adduct formation with human β-tubulin. Left, overall view showing the docked position of WA (in green), WE (in cyan), or WLA (in yellow). Right, enlarged view shows that WE and WLA cannot be docked into the WA-binding pocket.