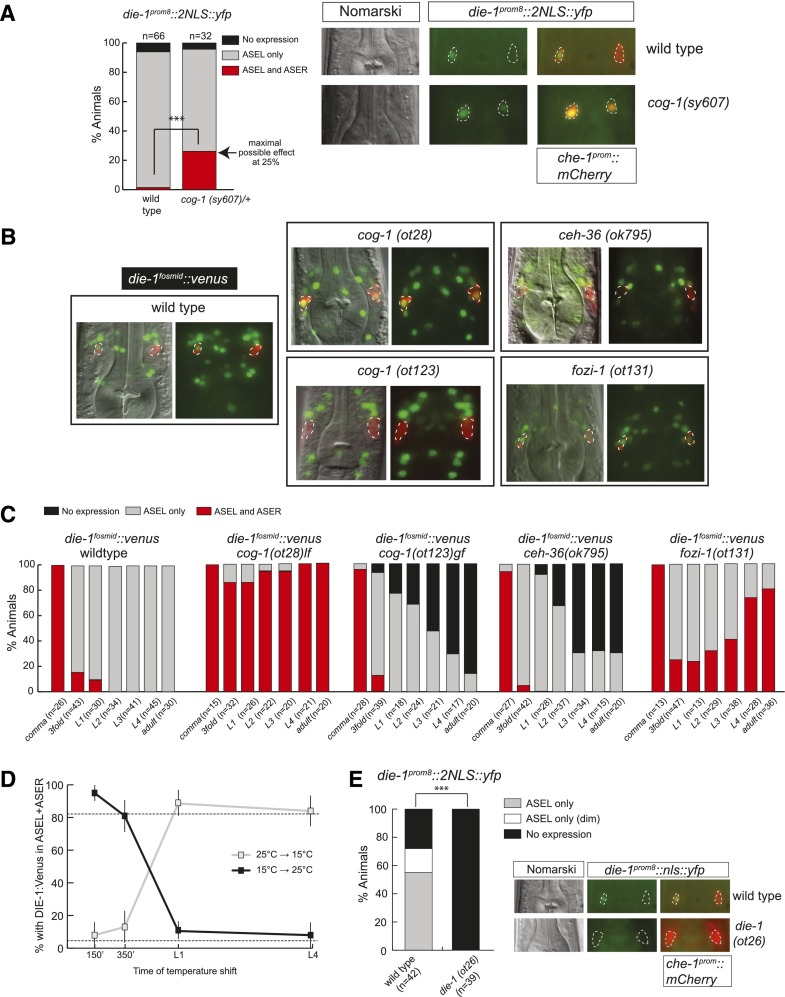
Figure 3.
Different phases of asymmetric die-1 expression in ASEL are controlled by distinct trans-acting factors. (A) cog-1 restricts die-1 expression at the transcriptional level. The die-1 prom8∷2nls∷yfp transcriptional reporter (otEx4359) (Fig. 1C) shows derepression in ASER in the background of a strong loss-of-function allele of cog-1(sy607). This allele is maintained as a heterozygote, as it has very low viability in homozygosity. Thus, the progeny of heterozygous mothers were analyzed. These should produce one-fourth of homozygous cog-1(sy607) progeny, and thus 25% of animals showing expression in ASEL+ASER suggest a fully penetrant derepression of die-1 in ASER in cog-1(sy607). Representative images are shown in which the ASE neurons are marked by che-1prom∷mCherry. (B) Representative images of L4/young adult animals with die-1∷2xFlag∷Venus (otIs274) fosmid expression in different ASE asymmetry mutant backgrounds. Animals contain che-1prom∷mCherry in the background to facilitate identification of the ASE neurons. Images were derived by Z-projections of stacks with focal planes showing die-1fosmid∷ 2xFlag∷Venus and che-1prom∷mCherry signals. The identity of the ASE neurons was confirmed by combining Nomarski and ASE-specific che-1prom∷mCherry signals. The die-1∷2xFlag∷Venus was carefully assessed to be in the corresponding ASE neuron nucleus. Due to the aggregation of the mCherry protein, some neurons have low or little mCherry in the nucleus. The 2xFlag∷Venus-labeled functional DIE-1 transcription factor (derived from the fosmid-based die-1∷2xFlag∷Venus reporter) is located exclusively in the nucleus. Some neurons with little or no mCherry (derived from the transcriptional che-1prom∷mCherry reporter) in the nucleus show no or little “yellow” staining in the merged fluorescence channels. See C for quantification. (C) Time course of die-1∷2xFlag∷Venus (otIs274) expression in different ASE asymmetry mutant backgrounds during early development until adulthood. Due to the difficulty of handling sterile cog-1(sy607)-null mutants, we scored the cog-1(ot28) hypomorphic allele, which also shows fully penetrant defects. (D) cog-1 is only required shortly after the birth of the ASE neurons to restrict die-1 expression to ASEL. The die-1∷2xFlag∷Venus (otIs274) reporter was analyzed in the background of a temperature-sensitive allele of cog-1(ot221). All animals were scored as L4 or young adults. Animals were grown at either the permissive temperature (15°C) or the restrictive temperature (25°C) and shifted to the opposite temperature at the indicated times. For synchronization, embryos were extracted with bleach, and two-cell embryos were isolated and allowed to develop for 150 or 350 min. The remaining animals were arrested as L1 by hatching in the absence of food and then allowed to continue development until L4 in the presence of food. (E) die-1 is required for its own expression. The die-1 prom8∷nls∷yfp transcriptional reporter (otEx4359) (Fig. 1C) loses expression in ASEL in the background of a loss-of-function allele of die-1(ot26). Representative images are shown in which the ASE neurons are marked by che-1prom∷mCherry. Animals were scored as L4 or young adults.