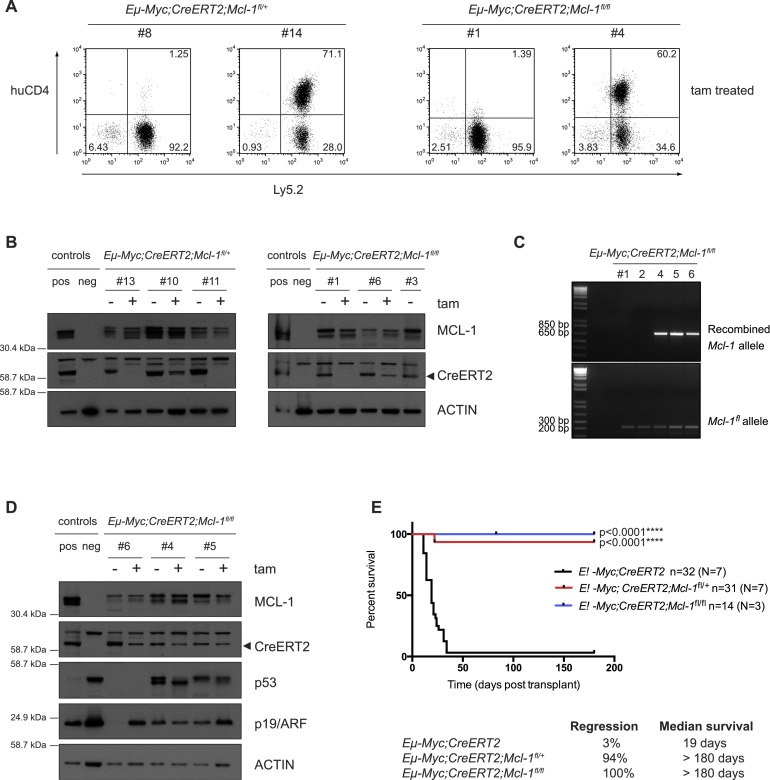
Figure 3.
Most Eμ-Myc lymphomas that relapse following tamoxifen treatment have escaped Mcl-1fl allele recombination. (A) FACS analysis to detect expression of huCD4 (reporter for Mcl-1fl recombination) on the surface of two representative Eμ-Myc;CreERT2;Mcl-1fl/+ and Eμ-Myc;CreERT2;Mcl-1fl/fl lymphomas that had relapsed in recipient mice after tamoxifen treatment (transplanted lymphoma cells stained positive for Ly5.2). Approximately 60% of the relapsed Eμ-Myc;CreERT2;Mcl-1fl/+ and 40% of the relapsed Eμ-Myc;CreERT2;Mcl-1fl/f lymphoma cells had escaped Mcl-1fl allele recombination and, as such, were huCD4-negative (like the sample at the left of each pair), whereas ∼40% of the relapsed Eμ-Myc;CreERT2;Mcl-1fl/+ and 60% of the relapsed Eμ-Myc;CreERT2;Mcl-1fl/fl lymphomas had efficiently recombined at least one Mcl-1fl allele, as reflected by staining positive for huCD4 (like the sample at the right of each pair). (B) Immunoblotting to detect MCL-1 and CreERT2 protein expression (probing for Actin was used as a loading control) in extracts from the spleens, lymph nodes, or thymuses of sick mice that had been transplanted with Eμ-Myc;CreERT2;Mcl-1fl/+ or Eμ-Myc;CreERT2;Mcl-1fl/fl lymphomas. Three independent, paired control, and tamoxifen-treated tumors of each genotype were analyzed (note that one of the Eμ-Myc;CreERT2;Mcl-1fl/fl tumors [#3] examined never relapsed after tamoxifen treatment). Mcl-1 knockout (KO) mouse embryonic fibroblasts (MEFs) were used as a control to confirm the specificity of the MCL-1 antibody (neg control). (C) DNA PCR analysis of recombined Mcl-1fl and unrecombined (intact) floxed alleles in Eμ-Myc;CreERT2;Mcl-1fl/fl lymphomas that relapsed following tamoxifen treatment. (D) Immunoblotting to detect MCL-1, CreERT2, p53, p19/ARF and Actin (loading control) protein expression in extracts from the spleens, lymph nodes, or thymuses of sick mice transplanted with Eμ-Myc;CreERT2;Mcl-1fl/fl lymphomas that relapsed as huCD4-positive following tamoxifen treatment. Three independent, paired control, and tamoxifen-treated tumors were analyzed. Mcl-1 knockout (KO) MEFs were used as a control (neg control) to confirm the specificity of the MCL-1 antibody. (E) Survival curves of C57BL/6-Ly5.1+ recipient mice transplanted with Eμ-Myc;CreERT2;Mcl-1fl/+ (red line) or Eμ-Myc;CreERT2;Mcl-1fl/fl (blue line) lymphoma cells that had wild-type p53 genes following tamoxifen treatment and excluding those lymphomas that escaped Cre-mediated deletion of one or both Mcl-1fl alleles. C57BL/6-Ly5.1+ recipient mice transplanted with control (Eμ-Myc;CreERT2; black line) lymphoma cells and treated with tamoxifen are shown for comparison. (n) Total number of recipient mice analyzed; (N) number of independent lymphomas tested. Efficient heterozygous and homozygous deletion of Mcl-1 significantly delayed lymphoma growth. (****) P < 0.0001. For the mice transplanted with the control Eμ-Myc;CreERT2 lymphomas, 3% regressed, and the overall median survival was 19 d. For the mice transplanted with the Eμ-Myc;CreERT2;Mcl-1fl/+ lymphomas, 94% regressed, and the overall median survival was >180 d. For the mice transplanted with the Eμ-Myc;CreERT2;Mcl-1fl/fl lymphomas, 100% regressed, and the overall median survival was >180 d.