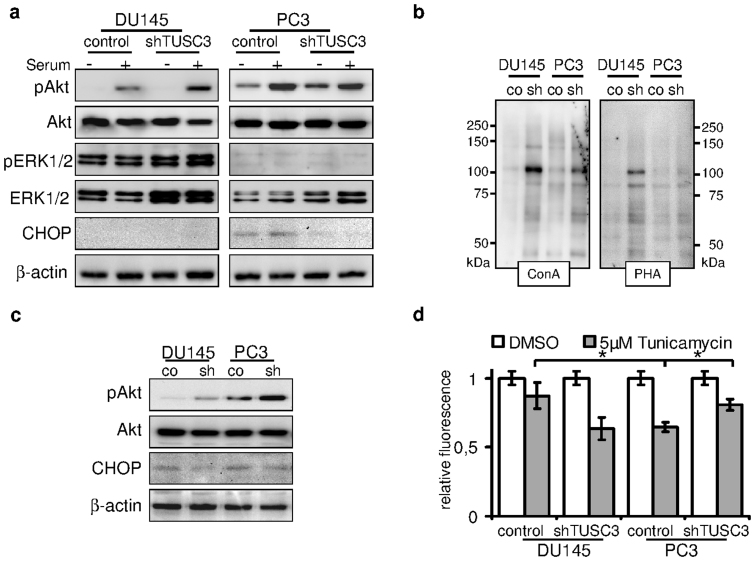
Figure 3. TUSC3 loss leads to increased viability, N-glycosylation and Akt signaling.
(a) shTUSC3 and control cells were serum starved for 36 hours (−) and serum was added for 30 minutes before lysis (+). PI3K/Akt and MAPK signaling pathway were evaluated by immunoblotting. Increased downstream activation of Akt can be observed in serum starved TUSC3 silenced PC3 cells as well as in DU145 cells after stimulation with serum. ER stress and CHOP are induced by prolonged serum starvation (lane 5 and 6) in PC3 cells. Loss of TUSC3 decreases CHOP levels in PC3 cells (lane 7 and 8). (b) Increased N-glycosylation in shTUSC3 cells. Lectin blotting using Concanavalin A and Phytohaemagglutinin-L lectins on isolated cell surface proteins was performed in cell membrane fractions of PC3 and DU145 cells following 72 h serum starvation. Control for protein loading was performed by amido black staining (Supplementary Figure S2C). (c) DU145 and PC3 prostate cancer cell lines were serum starved for 72 hours before lysis. Silencing of TUSC3 (sh) leads to sustained phosphorylation of Akt and decreased expression of CHOP in both cell lines. (d) Viability of TUSC3 silenced (shTUSC3) and control (scrambled shRNA) prostate cancer cells was assessed with the CellTiter-Blue® Assay after treatment with 5 μM tunicamycin or DMSO for 72 hours in full medium. Experiments were performed in triplicates and results are representative of several independent experiments. * p = 0.01.