Figure 2. Ribbon representation of Mtb CarD.
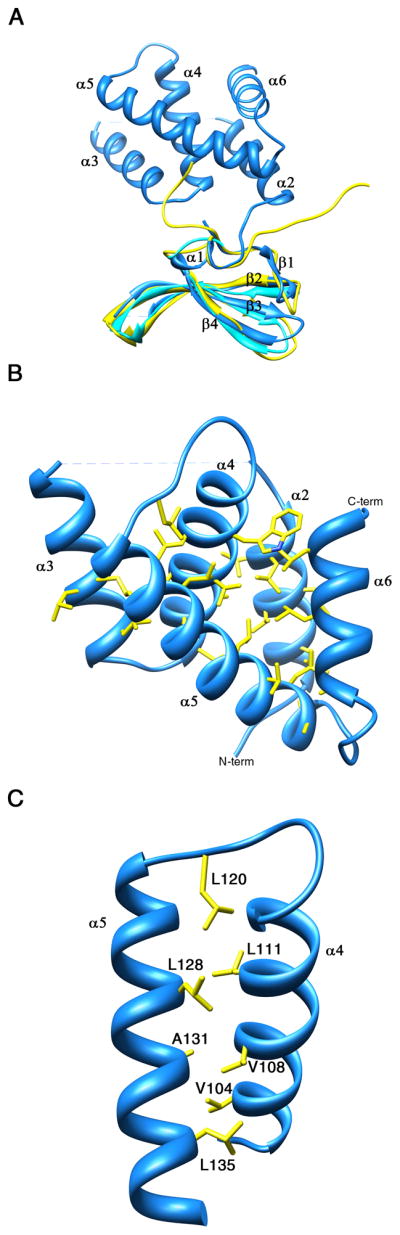
(A) Superposition of Mtb CarD N-terminal domain structure (blue) with the Tth CdnL-NMR structure (yellow) and the Tth TRCF-RID structure (cyan). The RNAP interacting β4-strand is labeled. The RMSD over the Cα backbone is 1.1 Å. (B) Ribbon representation of the CarD C-terminal domain. The helices contain mostly hydrophobic amino acids at their helix-helix interfaces, generating a hydrophobic core. The remainder of the structure is omitted for clarity. (C) The leucine zipper present between α4 and α5 in the C-terminal domain of CarD is formed by residues Leu120, Leu111, Leu128, Leu135, and surrounded by hydrophobic residues Val104, Val108 and Ala131. The rest of the structure is omitted for clarity. The leucine zipper lies inside the hydrophobic core of CarD and is not involved in dimerization or DNA interaction. In both panels, hydrophobic residues are shown with yellow sticks. See also Figure S1.
