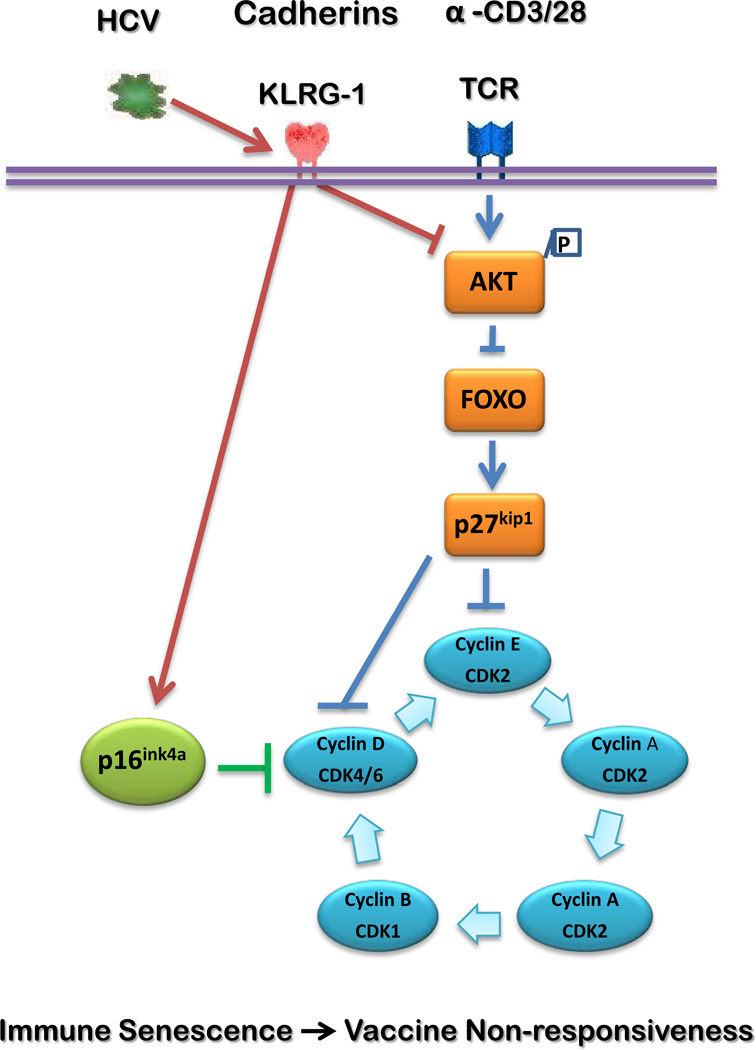
Fig. 6. A putative model for HCV-induced, KLRG1-mediated CD4+ T cell dysfunction via p16ink4a and p27kip1 pathways.
HCV-driven up-regulation of KLRG1 expression on CD4+ T cells inhibits TCR-induced Akt phosphorylation. This in turn lifts the block on forkhead box O (FOXO) transcription factors and activates p27kip1, causing G1 growth arrest by blocking the activations of cyclins and cyclin-dependent kinases (CDKs) [39, 40]. HCV infection also up-regulates p16ink4a expression in CD4+ T cells, which is linked to KLRG1 signaling. This in turn blocks the activations of Cyclins and CDKs, causing G1 growth arrest [1]. This KLRG1-mediated p16ink4a/p27kip1 alteration inhibits CD4+ T cell responses that are likely involved in the vaccine responses during chronic HCV infection. Therefore, blocking KLRG1 and its downstream signaling molecules may provide a novel approach to boosting vaccine responses in virally infected individuals.