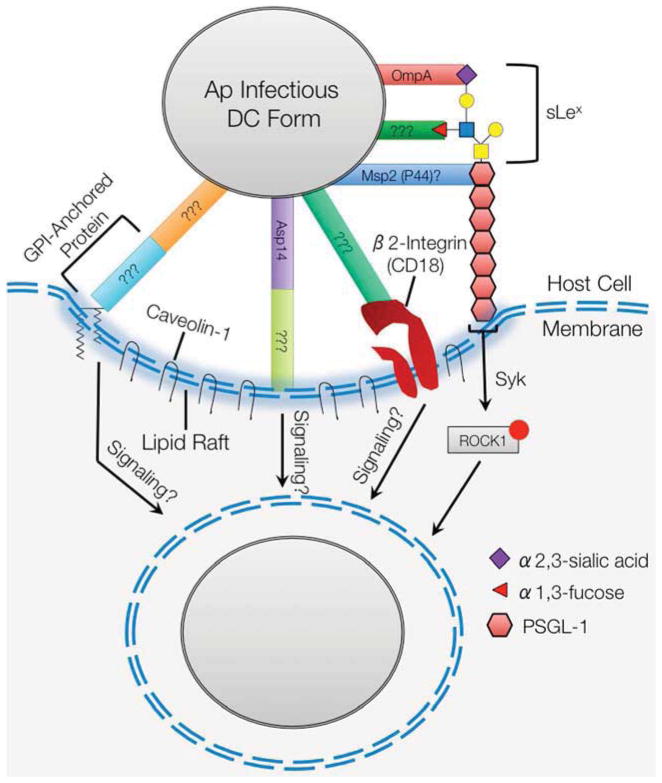
Figure 2.
A. phagocytophilum cellular invasion. The infectious dense-cored form of the bacterium utilizes multiple surface proteins to cooperatively bind PSGL-1 at an N- terminal amino acid sequence and α2,3-sialic acid and α1,3-fucose of the sLex tetrasaccharide that caps PSGL-1. Binding to PSGL-1 initiates a signaling cascade that involves Syk and phosphorylation of ROCK1 and promotes bacterial internalization. A. phagocytophilum also binds β2-integrin, but the relevance of this interaction is only detectable under conditions of shear flow. The pathogen binds at lipid rafts enriched for caveolin-1. Bacterial adherence to and infection of host cells from which GPI-anchored proteins have been cleaved is significantly impaired. OmpA, specifically a domain contained with amino acids 19–74, binds to α2,3-sialic acid and is the only A. phagocytophilum invasin for which a receptor is known. Asp14, specifically a domain contained with amino acids 100–124, is also important for invasion. Msp2 (P44), or a least a paralog thereof, may be involved in binding to PSGL-1. ???, unidentified A. phagocytophilum adhesins/invasins or host cell receptors.