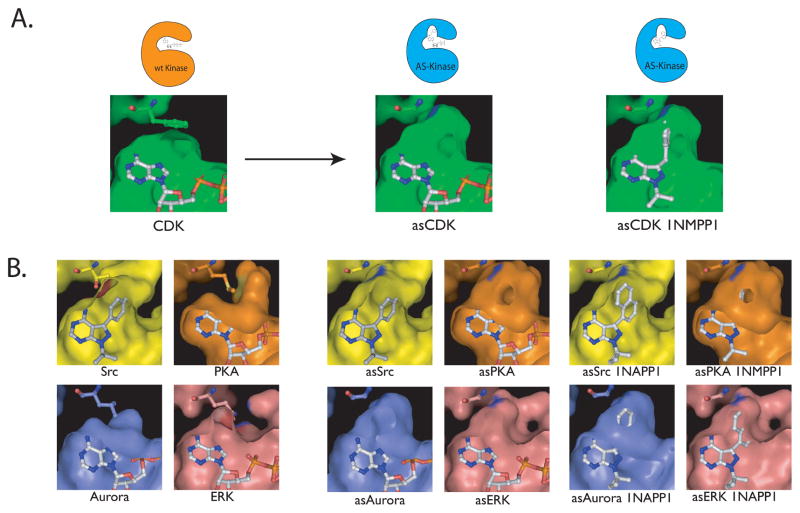
Figure 3.
A. The gatekeeper residue is shown in CDK1 as a phenylalanine above the bound ATP in the CDK1 active site. After mutation to the much smaller glycine, a pocket is opened that allows for a bulky substituent to bind (pictured as asCDK1). A “bumped” (N6 substituted) ATP analog or inhibitor (in this case 1NMPP1) can now bind to the engineered kinase by utilizing the extra space above the N7 position. B. The active sites of SRC, PKA, aurora and ERK are shown along with their gatekeeper residues and the successful mutation of these bulky residues to smaller ones. The mutation of the gatekeeper to a smaller residue creates a similar pocket in each of these four kinases. These kinases are shown with ATP bound or with bumped inhibitors that target these engineered kinases, but not the wild type. The similarity in the engineered pocket among disparate kinases demonstrates the wide applicability of this method.