Abstract
The main fast-acting inhibitory receptors in the mammalian brain are γ-aminobutyric acid type-A (GABAA) receptors for which neurosteroids, a subclass of steroids synthesized de novo in the brain, constitute a group of endogenous ligands with the most potent positive modulatory actions known. Neurosteroids can act on all subtypes of GABAA receptors, with a preference for δ-subunit-containing receptors that mediate extrasynaptic tonic inhibition. Pathological conditions characterized by emotional and motivational disturbances are often associated with perturbation in the levels of endogenous neurosteroids. We studied the effects of ganaxolone (GAN)—a synthetic analog of endogenous allopregnanolone that lacks activity on nuclear steroid receptors—on the mesolimbic dopamine (DA) system involved in emotions and motivation. A single dose of GAN in young mice induced a dose-dependent, long-lasting neuroplasticity of glutamate synapses of DA neurons ex vivo in the ventral tegmental area (VTA). Increased α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA)/N-methyl-D-aspartate ratio and rectification of AMPA receptor responses even at 6 days after GAN administration suggested persistent synaptic targeting of GluA2-lacking AMPA receptors. This glutamate neuroplasticity was not observed in GABAA receptor δ-subunit-knockout (δ-KO) mice. GAN (500 nM) applied locally to VTA selectively increased tonic inhibition of GABA interneurons and triggered potentiation of DA neurons within 4 h in vitro. Place-conditioning experiments in adult wild-type C57BL/6J and δ-KO mice revealed aversive properties of repeated GAN administration that were dependent on the δ-subunits. Prolonged neuroadaptation to neurosteroids in the VTA might contribute to both the physiology and pathophysiology underlying processes and changes in motivation, mood, cognition, and drug addiction.
Keywords: ganaxolone, tonic inhibition, AMPA receptors, dopamine neurons, δ-subunit-knockout mice, aversive conditioning
INTRODUCTION
Neurosteroids are a subclass of steroids synthesized de novo in the brain or through metabolism of precursors, such as progesterone leading to allopregnanolone, and remain one of the most potent endogenous positive modulators known for γ-aminobutyric acid type-A (GABAA) receptors (Lambert et al, 2009; Zorumski et al, 2013). Administration of neurosteroids acting on GABAA receptors induces anxiolytic, analgesic, anticonvulsant, sedative, and anesthetic effects (Lambert et al, 2003). Neurosteroids act on the GABAA receptor at distinct sites not overlapping with the binding sites of GABA, benzodiazepines, or barbiturates (Gee et al, 1995). Several conserved amino-acid residues responsible for their effects have been located in recombinant GABAA receptor α- and β-subunits (Hosie et al, 2006). Interestingly, the effects of neurosteroids are often abolished in mice deficient in δ-subunit-containing GABAA receptors (Mihalek et al, 1999), which are primarily located peri- and extrasynaptically (Nusser et al, 1998). At high micromolar concentrations, neurosteroids can affect both synaptic and extrasynaptic GABAA receptors (Lambert et al, 2009). Further, neurosteroid effects on GABAA receptors have been suggested to involve intracellular effects, such as protein kinase C activity (Fancsik et al, 2000). The mechanisms of neurosteroid actions on GABAA receptors, and especially the receptor subtypes responsible for specific behaviors, are presently poorly known.
Endogenous neurosteroids are powerful mood and motivation modulators in animals and humans (Backstrom et al, 2011; Lambert et al, 2009; Zorumski et al, 2013). Neurosteroids may also strongly influence responses to drugs of abuse. Allopregnanolone exerts an attenuating effect on cocaine-seeking behavior (Anker et al, 2010) and alters alcohol intake (Sinnott et al, 2002). Further, finasteride, an inhibitor of neurosteroid synthesis, may fully abolish anticonvulsant and inhibitory effects of alcohol (VanDoren et al, 2000). Growing evidence also suggests direct modulation of the mesocorticolimbic dopamine (DA) system by neurosteroids.
The mesocorticolimbic DA neurons projecting from the ventral tegmental area (VTA) constitute an important reinforcing pathway involved in the early actions of drugs of abuse, including those of GABAA receptor ligands (Heikkinen et al, 2009; Saal et al, 2003). The VTA DA neurons receive a wide variety of inputs from diverse brain regions, and their activity is ultimately regulated by GABAergic inhibition (Luscher and Malenka, 2011). In some studies, neurosteroids have acutely increased DA levels in the terminal fields of the mesolimbic pathway, namely, in the nucleus accumbens (Kalivas et al, 1990). However, place-conditioning studies have yielded contradictory results. Chronic allopregnanolone administration may lead to either rewarding (Finn et al, 1997) or aversive conditioning (Beauchamp et al, 2000). The mechanisms by which potent neurosteroids modulate VTA neurons are currently unknown. Elucidating the molecular and cellular basis of neurosteroid actions in the VTA DA pathway would enhance our understanding of behavioral changes in mood, motivation, learning, and addiction, all of which are modulated by altered activity of the mesocorticolimbic DA system.
Here, we have studied ganaxolone (GAN), a synthetic analog of allopregnanolone lacking nuclear steroid hormonal activity (Carter et al, 1997). We demonstrate for the first time that a single dose of a neurosteroid induces a long-lasting glutamate receptor neuroplasticity of DA neurons in the VTA, and that GAN in vitro increases tonic inhibition of local VTA GABA interneurons. We also used place-conditioning experiments in C57BL/6J mice and in GABAA receptor δ-subunit-knockout (δ-KO) mice to determine role of the δ-subunit-containing GABAA receptors in rewarding/aversive properties of GAN.
MATERIALS AND METHODS
Animals
For electrophysiological and behavioral GAN dose-testing assays, we used male and female 3–4-week-old transgenic Th-EGFP (Gong et al, 2003), GAD67-GFP knockin mice (Tamamaki et al, 2003), and GABAA receptor δ-KO mice and their wild-type littermates (δ-WT; Mihalek et al, 1999). Behavioral experiments were carried out with 8–10-week-old male C57BL/6J mice (Charles River, Sulzfeld, Germany) and male δ-KO and δ-WT littermate mice. All mice were group-housed under 12-h light/dark cycle with food and water available ad libitum. All experimental procedures in mice were approved by the Southern Finland Provincial Government.
Electrophysiological Experiments
For electrophysiological studies, the mice were naive or injected intraperitoneally (i.p.) between 0800 and 0900 hours with either GAN or a comparable volume of vehicle, and decapitated 24 h or 6 days later. Horizontal 225-μm-thick midbrain slices were prepared and preincubated as described previously (Heikkinen et al, 2009). In experiments involving in vitro drug exposure, slices were incubated with test substances for 3–6 h and after that they were transferred to regular aCSF for complete washout of the drug before recordings.
Electrophysiological recordings
Synaptic responses were measured using a whole-cell patch-clamp technique. Both VTA DA and GABA neurons were visualized using a fluorescence microscope (Olympus BX51WI, Hamburg, Germany). In δ-KO and δ-WT mice, the DA neurons were identified if a clear Ih current was observed after voltage clamping cells from −70 to −120 mV in 10 mV steps (Heikkinen et al, 2009). The currents were amplified (Multiclamp 700A, Molecular Devices, Sunnyvale, CA, USA), low-pass filtered at 1.6 kHz, and digitized at 20 kHz (Molecular Devices). Electrodes had a resistance of 3–5 MΩ when filled as follows (in mM, pH adjusted to 7.2–7.25, osmolarity to 280 mOsm): 130 cesium methanesulfonate, 10 HEPES, 0.5 EGTA, 8 NaCl, 5 QX314, 4 MgATP, 0.3 MgGTP, and 10 BAPTA for excitatory postsynaptic current (EPSC) recordings (Heikkinen et al, 2009); and 140 CsCl, 1 MgCl2, 10 HEPES, 0.1 EGTA, 4 NaCl, 2 MgATP, and 0.3 NaGTP for recordings of tonic currents and spontaneous inhibitory postsynaptic currents (sIPSC; Glykys et al, 2008). Access and membrane resistances were monitored throughout the experiment, and the recording was discarded if the access resistance changed >20% during the experiment.
AMPA/NMDA receptor current ratio
α-Amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) and N-methyl-D-aspartate (NMDA) receptor EPSCs were induced by stimulating glutamatergic afferents at 0.1 kHz frequency using a bipolar stimulus electrode (Vashchinkina et al, 2012). Neurons were clamped at +40 mV in the presence of GABAA receptor blocker picrotoxin (100 μM), and EPSCs were recorded for 10 min before and after the application of NMDA receptor blocker D-(−)-2-amino-5-phosphonopentanoic acid (AP5 50 μM). The ratio was calculated by dividing the peak amplitude of AMPA receptor current with that of NMDA receptor current, averaged from 30 EPSCs.
Rectification index
Recordings were carried out using freshly made spermine (100 μM) in the pipette solution, and AMPAR-EPSCs were evoked at holding potentials of −70 mV and +40 mV in the presence of picrotoxin (100 μM) and AP5 (50 μM; Dingledine et al, 1999). The rectification index was calculated as the ratio between evoked AMPAR-EPSC peak amplitudes at −70 mV and those at +40 mV.
Tonic current and sIPSC measurement
VTA DA and GABA neurons were voltage-clamped at −70 mV. GABAA receptor-mediated sIPSCs were recorded in the presence of kynurenic acid (4 mM) and CGP 35348 (200 μM) at 32–34 °C. Series resistance and whole-cell capacitance were estimated and compensated by 70% (lag 7 μs). GABA (500 nM), GAN (500 nM), GABA transporter blocker NO-711 (10 μM), and GABAA receptor blocker gabazine (100 μM) were applied directly to the neurons for at least 5 min using a Y-tube focal applicator controlled by a Picopump PV820 (WPI, Sarasota, FL, USA). Recorded events were filtered at 2 kHz, digitized at 20 kHz, and analyzed automatically with Mini Analysis software (Synaptosoft). For sIPSC kinetics, an average of 300–500 events per cell and condition were scaled and fit with double exponential I(t)=A1(exp(−t/τ1))+A2(exp(−t/τ2)), with A1 and A2 as the amplitudes for fast and slow components and τ1 and τ2 as their respective time constants. Tonic GABAA-mediated current was defined as the shift in inward holding current after application of the GABAA blocker gabazine (Semyanov et al, 2003) and measured as described by Glykys and Mody (2007) using Clampfit software (pClamp 10, Molecular Devices). The mean current values were obtained from Gaussian fits of all-point amplitude histograms of current. Histograms were constructed from 5 to 10 s of data with a bin width of 1 pA using Prism 5.0 software (GraphPad Software, San Diego, CA, USA), with the points falling on sIPSCs being discarded.
Behavioral Experiments
Spontaneous locomotor activity
Effects of GAN (10 and 30 mg/kg) and vehicle on locomotor activity were tested for 90 min immediately after acute injection (i.p.). Young mice were adapted to the experimental room for 2 h, after which their locomotor activities were monitored in Plexiglas cages (45 × 22.5 × 15 cm; Tecniplast, Buguggiate, Italy) by an Ethovision Color-Pro 3.1 video-tracking system (Noldus Information Technology, Wageningen, The Netherlands; Heikkinen et al, 2009).
Place-conditioning tests
The procedure was performed in eight Plexiglas cages covered with transparent lids with ventilation holes (Cunningham et al, 2006; Vashchinkina et al, 2012). Two types of preselected floor material (plastic 1.2-cm-wide flat bars separated by 0.5-cm gaps and a metal grid with 1-mm-spacing between wires) were used as conditioning stimuli. During 15-min preliminary tests for material preference, naive mice showed no preference for either one of the floor materials (data not shown). Therefore, we followed the unbiased conditioned place preference (CPP) procedure (Cunningham et al, 2006). Three independent batches of adult mice were tested.
In the habituation phase, the mice were weighed, injected with vehicle, and placed into conditioning cages. The conditioning phase consisted of 30-min presentations to floor materials differentially paired with GAN and vehicle pretreatments. The mice were randomly assigned to one conditioning subgroup (metal+ or metal−). The metal+ subgroup received GAN paired with the metal floor (conditioning stimulus+ (CS+) trial) and vehicle with the plastic floor (CS−). The metal− subgroup received GAN paired with the plastic floor (CS+) and vehicle with the metal floor (CS−). Mice received four CS+ and four CS− trials on alternating days (eight trials in total). Each group was counterbalanced for the direction of the floor materials inside the cages and for the order that the trials were presented.
The place preference test (30 min) was performed 24 h after the last conditioning trial. The mice were habituated to the experimental room for 1 h before the testing. The cage floor was covered half and half with plastic and metal materials, bisecting it into two distinct zones on which the mice could move according to their preference. The animals were injected with vehicle and placed in the center of the cage, with orientations of the materials being counterbalanced within each group. Time spent on the metal grid was used as the primary dependent variable. Locomotor activities and locations were determined by Ethovision. Between the trials, all cages and floor materials were thoroughly washed with water and dried to remove odors. The experiments were performed between 0800 and 1700 hours.
Anxiolytic effects of 30 mg/kg GAN on naive δ-KO and δ-WT were estimated during the first conditioning session, for the first 15 min until GAN-induced sedation appeared. Anxiety-related behaviors were evaluated as percentage of total distance moved at the center of the arena.
Body temperature measurement
To determine possible hypothermic effects of the drugs, naive adult male C57BL/6J and δ-KO mice were used. Mice were habituated to handling and injections for 2 days. Ambient temperature was 20±0.5 °C. Immediately before drug administration, baseline temperature was measured by inserting a rectal thermometer probe (Physitemp Instrument Inc., Clifton, NJ, USA). Thereafter, body temperature was recorded at different intervals (ranging from 15 to 30 min) after an i.p. injection of vehicle, GAN (30 mg/kg), or 4,5,6,7-tetrahydroisoxazolol[4,5-c]pyridine-3-ol (THIP, gaboxadol; 6 mg/kg), a selective GABA-site agonist with a full agonist profile at δ-subunit-containing GABAA receptors (Storustovu and Ebert, 2006). THIP has shown to induce neuroplasticity in VTA DA neurons but no reward-like behavior in CPP test in mice (Vashchinkina et al, 2012).
Measurement of DA Levels
DA and metabolites were measured by HPLC as described elsewhere (den Hollander et al, 2013). Striatal and frontal cortices were dissected from nine adult male Th-EGFP and nine littermate wild-type mice and stored at −80 °C until analysis. Samples were prepared by sonication in 10 volumes of 2% perchloric acid and centrifuged for 30 min at 15 000 g, after which 10 μl of filtered supernatant was injected into the HPLC system. Results were normalized by wet brain weight.
Drugs
GAN ((3α,5α)-3-hydroxy-3-methyl-pregnan-20-one, Tocris Bioscience, Bristol, UK) was dissolved in 20–30% (2-hydroxypropyl)-β-cyclodextrin (Sigma-Aldrich, St Louis, MO, USA) by sonication for injections, or in DMSO (0.1%) for in vitro experiments. THIP (4,5,6,7-tetrahydroisoazolo(5,4-c)pyridin-3-ol hydrochloride donated by Bjarke Ebert, H Lundbeck A/S) was dissolved in 0.9% NaCl solution (saline). All stock solutions for mice were prepared on the day of treatment and injected at a volume of 100 and 200 μl for adult and adolescent mice, respectively, per 10 g of body weight. Spermine, NO-711, GABA, and gabazine (SR-95531) were purchased from Sigma-Aldrich. Stock solutions of kynurenic acid sodium salt (Abcam PLC, Cambridge, UK), picrotoxin, AP5, and CGP 35348 (all from Tocris Bioscience) were diluted in ACSF and added to perfusion medium when needed.
Statistical Analyses
Statistical analysis was carried out using Prism 5.0 software (GraphPad Software, La Jolla, CA, USA) and SPSS 15.0 software (SPSS Inc, Chicago, IL, USA). Statistical significance of the differences between data groups with equal variances was assessed with t-test or with one-way or two-way ANOVA followed by Bonferroni post-test (P<0.05).
RESULTS
Effects of GAN on Locomotor Activity of Mice
We first searched for slightly sedative doses of GAN after acute injections to locate the closest match to our previous study with THIP (Vashchinkina et al, 2012) that has a full agonistic profile at extrasynaptic GABAA receptors (Storustovu and Ebert, 2006). We found that GAN 30 mg/kg produced a transient reduction in locomotor activity of young mice, whereas a 10 mg/kg dose did not affect activity (Figure 1a and b). A 1-h measure of cumulative locomotor activities was decreased only by the higher dose of GAN as compared with vehicle (Figure 1b; F2,67=3.52, P<0.05). The GAN-reduced activity returned to baseline by 60 min after the injections (Figure 1a). No sex differences were present in locomotor activity after the GAN treatment (cumulative distance: t1,22=0.86, P>0.05).
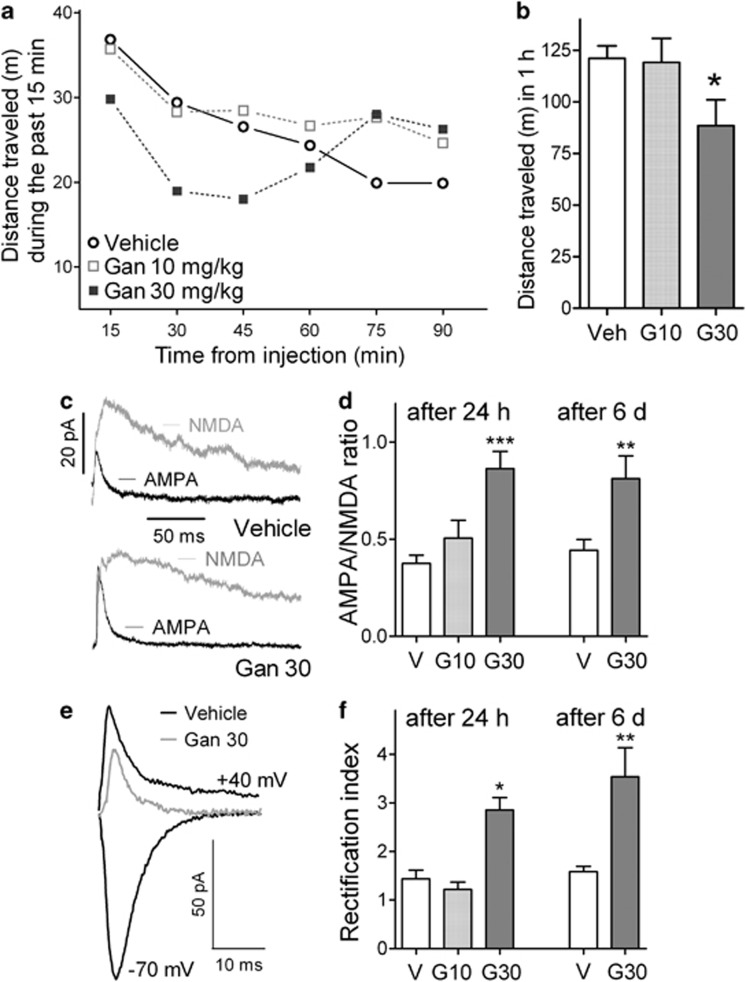
Figure 1.
Single dose of ganaxolone (GAN, 30 mg/kg) increased the α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA)/N-methyl-D-aspartate (NMDA) receptor current ratio and the rectification index in ventral tegmental area (VTA) dopamine (DA) neurons of midbrain slices obtained ex vivo 1–6 days after the drug administration. (a) Locomotor activity after acute injection of GAN (10 mg/kg, n=17; and 30 mg/kg, n=24) and vehicle control (n=30) in 3–4-week-old Th-EGFP mice. The data are given as the mean distance travelled for 15-min periods preceding the time points (SEM values are within the symbols). (b) Cumulative locomotor activity (mean+SEM) for 60 min after injection for the mice is shown in a. *P<0.05 for significance of difference from the vehicle group. (c) Examples of AMPA and NMDA receptor-mediated current traces for vehicle- and GAN (30 mg/kg)-treated young transgenic Th-EGFP mice observed 24 h after the injection. (d) Time course of the effects of vehicle and GAN (10 and 30 mg/kg) administration on AMPA/NMDA ratios of VTA DA neurons is shown as mean bars+SEM (n=7–11 mice), the effect by the higher GAN dose persisting at least 6 days after the injection. (e) Representative AMPA receptor-mediated current traces in the VTA DA neurons recorded at −70 and +40 mV 24 h after vehicle and GAN (30 mg/kg) administrations in Th-EGFP mice, in the presence of spermine in the recording pipette solution. Decreased currents at +40 mV after GAN suggest increased rectification because of insertion of new GluA2-lacking AMPA receptors. (f) Time course of the effects of vehicle and GAN (10 and 30 mg/kg) administration on the rectification index (response at −70 mV/response at +40 mV) of VTA DA neurons is shown as mean bars+SEM (n=9–11 mice), indicating persistence of the effect for at least 6 days. *P<0.05, **P<0.01, ***P<0.001 (ANOVA with Bonferroni test or Student's t-test).
Single In Vivo Doses of GAN Induce Dose-Dependent Long-Lasting Potentiation in the VTA DA Neurons Via Insertion of New Glu2-Lacking AMPA Receptors
To assess the synaptic strength of glutamatergic synapses in VTA DA neurons, we measured the AMPA/NMDA ratios. In VTA DA neurons of midbrain slices from GAN-treated mice obtained 24 h after the injection, the AMPA/NMDA ratios were significantly higher than that in neurons from vehicle-treated mice (Figure 1c and d). GAN produced a dose-dependent increase in the ratio, as the dose of 30 mg/kg was different from the vehicle values, whereas the 10 mg/kg dose was not. The effect of GAN administration was long lasting; a significant increase in the AMPA/NMDA ratio was evident at least 6 days after the single 30 mg/kg GAN injection (Figure 1d; treatment effect F1,35=45.74, P<0.001, two-way ANOVA). The controls examined at 1- and 6-day time points after the vehicle injection (n=7–11 mice per group) did not differ significantly from one another (Figure 1d; P>0.05).
To determine whether GAN induces potentiation in the VTA DA neurons through insertion of new GluA2 subunit-lacking receptors in a similar manner as THIP (Vashchinkina et al, 2012), we tested the I–V relationships of AMPAR-EPSCs and calculated the rectification index (Luscher and Malenka, 2011). The pipette solution containing polyamines selectively blocks the GluA2-lacking AMPA receptors (Dingledine et al, 1999), which can be seen as increased rectification at positive holding potentials. We found that the normalized currents at the +40 mV holding potential were decreased in VTA DA neurons obtained 24 h after the administration of 30 mg/kg GAN relative to those obtained from vehicle-treated mice (Figure 1e and f) and that the calculated rectification indices were higher for GAN-treated neurons. The rectification index was still significantly higher 6 days after the GAN injection (Figure 1f; treatment effect F1,28=41.14, P<0.001, two-way ANOVA). The effect of GAN 30 mg/kg was similar to the effect of THIP 6 mg/kg (Vashchinkina et al, 2012).
To ascertain that DA levels and metabolism were unaltered by the Th-EGFP transgene, we compared the levels of DA and its acidic metabolites (DOPAC and HVA) in striatal and frontocortical tissues between naive Th-EGFP and δ-WT mice. No differences were observed between the transgenic and littermate mice in DA or metabolite levels (in nmol/g wet weight, mean±SEM, n=9; striatal and frontal cortical DA: 15.7±2.4 and 0.32±0.1 vs 14.8±1.2 and 0.47±0.35, respectively, for Th-EGFP and non-Th-EGFP mice; striatal and frontal cortical DOPAC: 11.5±0.6 and 1.5±0.4 vs 12.0±0.9 and 1.6±0.4, respectively, for Th-EGFP and non-Th-EGFP mice; striatal and frontal cortical HVA: 4.4±0.3 and 1.6±0.3 vs 4.3±0.3 and 1.5±0.2, respectively, for Th-EGFP and non-Th-EGFP mice (genotype effect F5,96<0.03, P>0.05, two-way ANOVA)).
GAN Selectively Enhances Tonic Currents in VTA GABA Neurons and Induces Synaptic Plasticity Dependent on δ-Subunits
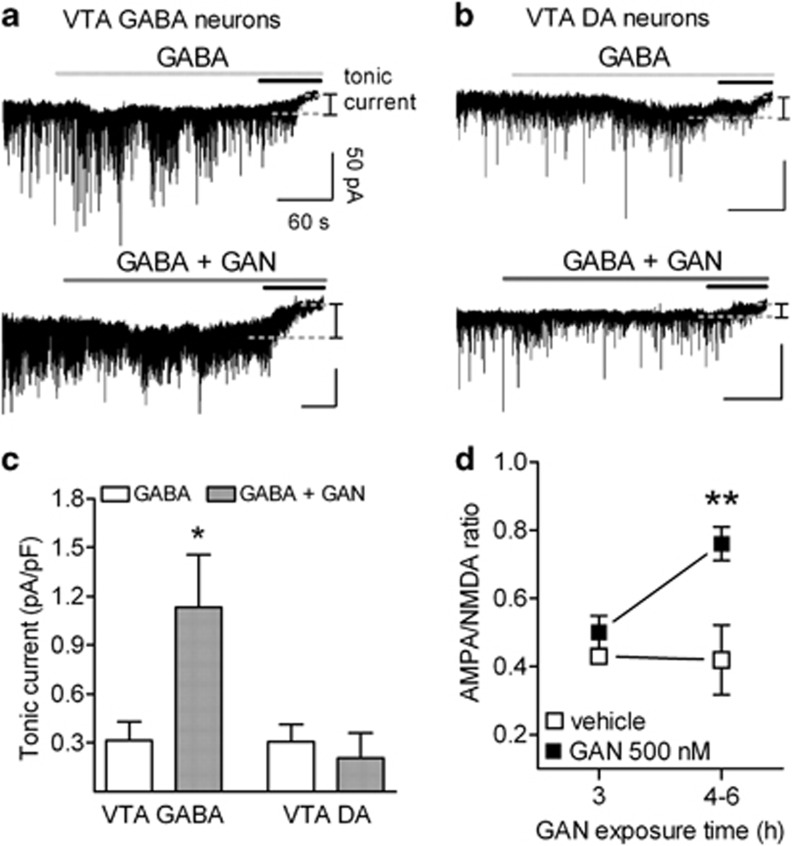
To assess the functional activity of δ-subunit-containing GABAA receptors in the VTA, we tested direct effects of GAN on the GABAA receptor-mediated currents in VTA DA neurons and local GABA interneurons in acute midbrain slices of Th-EGFP and GAD67-GFP mice, respectively. First, in the presence of 500 nM GABA and 200 μM GABA transporter inhibitor NO-711, the application of the GABAA receptor antagonist gabazine (SR-95531) blocked sIPSCs and led to a decrease in Ihold, reflecting the presence of a tonically activated current in both cells types (Figure 2). To correct for differences in cell size, the tonic current was normalized to the cell's capacitance (average capacitances were 71.8±6.9 pF (n=15) and 30.8±2.5 pF (n=15) for the VTA DA and GABA neurons (P<0.0001, unpaired Student's t-test), respectively). We next recorded tonic currents after the addition of 500 nM GAN to obtain functional evidence for tonic inhibition in VTA neurons. Local 4-min application of GAN directly to the cells selectively increased the tonic inhibition of the GABA neurons but not of the DA neurons (Figure 2a–c; cell type × treatment F1,29=4.80, P<0.05). Analysis of sIPSC events revealed that GAN did not affect the rise time, peak amplitudes, decay kinetics, or charge transfer in either DA or GABA neurons (Table 1). There was only a trend toward an increase in decay kinetics (τ1, τ2, and weighted τ) in the GABA neurons (Table 1), which is in line with previous findings on hippocampal and cerebellar granule cells (Stell et al, 2003). Our results clearly indicated that in the GABA neurons the tonic current was markedly increased, whereas the phasic current responses were not affected by the moderate concentration of GAN.
Figure 2.
Low concentrations of ganaxolone (GAN) selectively enhance the magnitude of tonic current in vitro in ventral tegmental area (VTA) γ-aminobutyric acid (GABA) neurons and induce an increase in α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA)/N-methyl-D-aspartate (NMDA) ratio within 4-h incubation in VTA dopamine (DA) neurons. (a) Traces obtained from VTA GABA neurons and (b) DA neurons. Horizontal lines indicate the local applications of 200 μM NO-711 with 500 nM GABA (light gray) or with 500 nM GABA+500 nM GAN (dark gray) and GABAA receptor antagonist gabazine at 100 μM (black). The dotted line is the mean current after the block of GABAA receptors used to calculate the magnitude of the tonic GABAA receptor-mediated current. This current was increased in the presence of 500 nM GAN in VTA GABA neurons. (c) Graph showing tonic current amplitudes recorded in VTA GABA and DA neurons. GAN selectively increased tonic current in the GABA cells. (d) Averaged AMPA/NMDA ratios in VTA DA neurons of midbrain slices incubated for 3–6 h in the presence of 500 nM GAN. The 4–6-h GAN incubation significantly increased the ratios compared with vehicle treatment and 3-h GAN incubation. Data are presented as means+SEM, n=6–9 mice per group. *P<0.05, **P<0.01 (ANOVA with Bonferroni test).
Table 1. Comparison of Parameters for GABAA Receptor-Mediated sIPSCs in VTA GABA and DA Neurons with and without Exogenous GABA and Ganaxolone (GAN).
| Cell type (no. of recorded cells) | Frequency (Hz) | Amplitude (pA) | Rise time (ms) | τ1 (ms) | τ2 (ms) | Weighted τ (ms) | Charge transfer (fC/s) |
|---|---|---|---|---|---|---|---|
| VTA GABA neurons | |||||||
| Control (20) | 4.6±0.9 | 52.7±9.1 | 1.2±0.1 | 3.1±0.3 | 14±3 | 6.7±0.8 | 5984±2930 |
| 500 nM GABA (9) | 8.5±2.4 | 55.5±8.7 | 1.3±0.1 | 3.7±0.8 | 24±9 | 8.5±1.6 | 6294±2566 |
| 500 nM GABA+500 nM GAN (11) | 5.5±1.2 | 59.1±13.4 | 1.0±0.1 | 5.1±1.2 | 28±14 | 9.9±2.6 | 4766±1517 |
| VTA DA neurons | |||||||
| Control (16) | 3.6±0.7 | 46.2±4.3 | 1.0±0.1 | 4.3±0.8 | 16±4 | 15.6±3.8 | 4264±1338 |
| 500 nM GABA (8) | 3.9±1.1 | 40.4±5.9 | 1.3±0.2 | 4.4±0.8 | 26±7 | 12.5±2.8 | 4065±1484 |
| 500 nM GABA+500 nM GAN (8) | 3.5±1.1 | 36.4±4.5 | 1.0±0.1 | 4.6±1.2 | 40±15 | 16.8±5.9 | 3685±2399 |
Values are means±SEM (n). No significant differences emerged from the corresponding control values within the neuronal populations (ANOVA).
To further confirm that tonic inhibition of VTA GABA neurons is sufficient to produce synaptic plasticity in VTA DA neurons, we exposed midbrain slices to GAN. Recordings performed after 4-h incubation of slices with 500 nM GAN revealed significant increase in AMPA/NMDA ratio in the VTA DA neurons compared with the control slices incubated in the presence of the vehicle (0.1% DMSO; Figure 2d; treatment effect F1,17=3.63, P<0.001, two-way ANOVA).
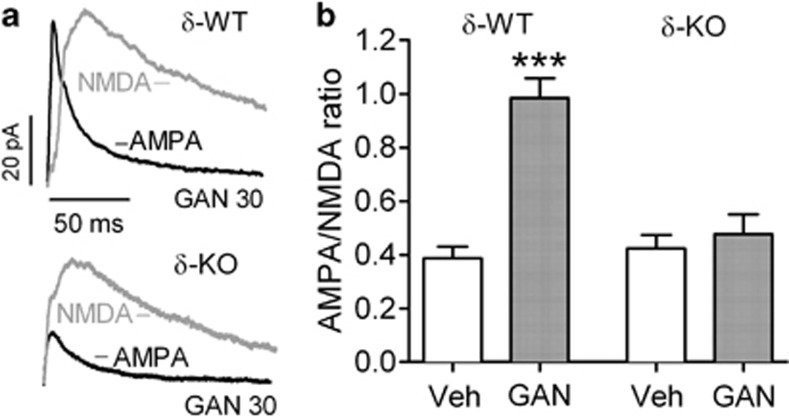
Finally, we tested whether GAN in vivo induces neuroplasticity in VTA DA neurons of δ-KO mice that are known to have reduced tonic extrasynaptic GABAA receptor-mediated inhibition (Stell et al, 2003) and reduced behavioral responses to GAN (Mihalek et al, 1999). In δ-KO mice, GAN 30 mg/kg given 24 h before preparing slices did not have an effect on AMPA/NMDA current ratio in the VTA DA neurons, in contrast to the increased ratio in δ-WT mice (Figure 3a and b; drug × genotype interaction F1,20=22.25, P<0.001). Neurons of δ-WT mice demonstrated increased AMPA/NMDA ratios after GAN administration similar to the transgenic Th-EGFP's neurons lacking Ih current (Figure 3; δ-WT vehicle 0.39±0.09 (n=5) and GAN 30 mg/kg 0.98±0.16 (n=5) vs Th-EGFP Ih(−) vehicle 0.36±0.03 (n=7) and GAN 30 mg/kg 0.86±0.11 (n=9); drug × mouse line interaction F1,22=0.04, P>0.05). Taken together, we conclude that GAN-induced synaptic plasticity depends on δ-subunit-containing GABAA receptors.
Figure 3.
Ganaxolone (GAN) failed to increase the α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA)/N-methyl-D-aspartate (NMDA) ratio in ventral tegmental area (VTA) dopamine (DA) neurons 24 h after the treatment in γ-aminobutyric acid type-A (GABAA) receptor δ-subunit-knockout (δ-KO) mice. (a) Representative examples of AMPA and NMDA receptor-mediated current traces after GAN 30 mg/kg for wild-type littermate (δ-WT) and δ-KO mice. (b) AMPA/NMDA receptor peak current ratios after vehicle and GAN treatments for δ-WT (vehicle, n=5, and GAN, n=5, where n is the number of tested mice) and δ-KO mice (vehicle, n=5, and GAN, n=9). Calibration: 20 pA/50 ms. Values are shown as means+SEM. ***P<0.001 (ANOVA with Bonferroni test).
GAN Induces a Dose-Dependent Conditioned CPA in C57BL/6J Mice
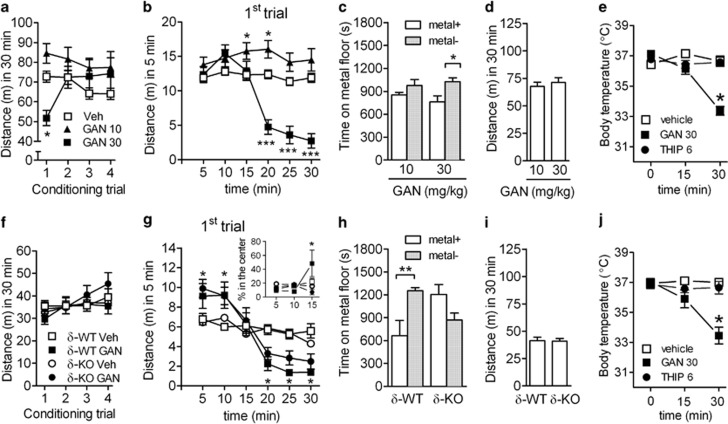
We recently found that although THIP induced neuroplasticity in VTA DA neurons, it failed to show reward-like behavior in a mouse-CPP test and in a baboon self-administration test (Vashchinkina et al, 2012). Therefore, we now tested using mice whether GAN can induce a CPP. Locomotor activity during the conditioning trials is shown in Figure 4a and b. During the vehicle trials, habituation occurred in both GAN dose groups (trial effect F3,111=3.45, P<0.05) equally (dose effect F1,37=0.92, P=0.34) and, thus, the vehicle trial data were collapsed across the dose groups (Figure 4a). When each conditioning trial was analyzed in 5-min time-bins, the 10 mg/kg dose of GAN slightly increased locomotor activity during the first conditioning trial in a period from 10 to 20 min (Figure 4b; time × dose × drug interaction F5,370=13.54, P<0.001). However, no effect was observed during the rest of the trials (data not shown). GAN at 30 mg/kg reduced locomotor activity during the first trial, an effect that was attributable to its strong sedative locomotor-decreasing effect on the latter half of the trial (Figure 4b; time × dose × drug interaction F5,370=13.54, P<0.001). No overall locomotor activity-decreasing effect was seen during the rest of the trials (trial × dose interaction F3,222=3.62, P<0.05), as the locomotor-decreasing effect was delayed in the rest of the trials and only occurred during the last 5 min of the trial (data not shown). In the test trials, the mice conditioned with 30 mg/kg GAN expressed significant place aversion (conditioned place aversion; CPA), whereas the mice conditioned with 10 mg/kg GAN showed neither preference nor aversion (Figure 4c; conditioning effect F1,35=9.92, P<0.01). The test trial locomotor activity was similar at the two doses (Figure 4d; t=−0.62, df=37, P=0.54).
Figure 4.
Place conditioning with ganaxolone (GAN) in adult C57BL/6J and δ-KO mice. (a–e) Results from experiments with C57BL/6J mice: (a) locomotor activity during each conditioning trial with two doses of GAN (conditioning stimulus (CS)+) and with vehicle (CS−) (vehicle trial data from both dose levels were combined). Conditioning time for each vehicle and active drug trial was 30 min. *P<0.05, compared with vehicle group (ANOVA with Bonferroni test). (b) Locomotor activity in 5-min time-bins during the first conditioning trial. *P<0.05 and ***P<0.001, compared with vehicle group. (c) C57BL/6J mice conditioned with GAN at a dose of 30 mg/kg expressed place aversion at 24 h after the last conditioning session, whereas those conditioned with the lower dose failed to express any preference or aversion. Test trial time after vehicle injection was 30 min. *P<0.05. (d) Locomotor activity during place conditioning test trial. Data for C57BL/6J mice are presented as means±SEM, n=14–25. (e) GAN-induced hypothermia in C57BL/6J mice after treatment with GAN 30 mg/kg, 4,5,6,7-tetrahydroisoxazolol[4,5-c]pyridine-3-ol (THIP) 6 mg/kg or vehicle. *P<0.05. (f–j) Results from experiments with γ-aminobutyric acid type-A (GABAA) receptor δ-subunit-knockout (δ-KO) mice and their WT littermates (δ-WT): (f) locomotor activity during each conditioning trial. GAN (30 mg/kg) conditioning was performed identically as with C57BL/6J mice. (g) Locomotor activity in 5-min time-bins during the first conditioning trial. The inset shows the percentage of total distance moved in the center of the arena during the first conditioning trial, indicating increased activity in the WT mice after GAN. *P<0.05, compared with the corresponding vehicle group (ANOVA with Bonferroni test). (h) WT mice expressed GAN-induced conditioned place aversion (CPA), whereas the KO mice displayed neither preference nor aversion. **P<0.01. (i) Locomotor activity during place-conditioning test trial. (j) GAN-induced hypothermia in δ-KO mice after treatment with GAN 30 mg/kg, THIP 6 mg/kg or vehicle. *P<0.05. Data for δ-WT and δ-KO mice are presented as means±SEM, n=7–11.
GAN-Induced Place Aversion is Absent in δ-KO Mice
We used the δ-KO mice to ascertain whether aversive properties of GAN are dependent on the δ-subunit-containing GABAA receptors. Locomotor activity during the conditioning trials is depicted in Figure 4f. During the vehicle trials (CS− trials), both genotypes had equal locomotor activity (trial × genotype interaction F3,105=0.68, P=0.57). When locomotor activity of the first conditioning trial was dissected into 5-min time-bins, as for the C57BL/6J mice (Figure 4b), a biphasic effect of 30 mg/kg GAN was discovered in δ-WT and δ-KO mice. Thus, 30 mg/kg GAN first increased then decreased the locomotor activity (Figure 4g; time × drug interaction F5,350=17.06, P<0.001). Interestingly, this effect was similar in both genotypes (time × genotype × drug interaction F5,350=0.59, P=0.71), and was also present throughout the rest of the conditioning trials (data not shown). In both δ-WT and δ-KO mice, a slight overall increase in the locomotor activity took place during vehicle (CS−) and GAN (CS+)-conditioning trials (Figure 4f; trial effect F3,210=5.62, P<0.01). Twenty-four hours after the last conditioning trial, the expression of GAN-induced place conditioning was measured after vehicle administration. The δ-WT mice expressed a significant place aversion, whereas no place conditioning was observed in δ-KO mice (Figure 4h; genotype × conditioning interaction F1,33=14.02, P<0.01). Locomotor activity during the place-conditioning test trial was equal between the genotypes (Figure 4i; t=−0.14, df=35, P=0.89).
During the first conditioning trial with GAN 30 mg/kg, δ-WT mice moved more in the center area of the conditioning cage in the time period from 10 to 15 min than δ-KO mice (Figure 4g inset; time × genotype × drug interaction F2,50=8.02, P<0.01).
GAN-Induced Hypothermia
Hypothermic effects of GABAA receptor agonists have been suggested to influence performance in a drug-induced CPP test (Cunningham and Niehus, 1993). In fact, neurosteroids induce strong hypothermia (Melchior and Allen, 1992), and we hypothesized that the 30 mg/kg dose of GAN would be associated with hypothermia, which might then result in aversive conditioning effects. The effects of GAN 30 mg/kg and, for comparison, THIP 6 mg/kg on rectal body temperature were assessed in C57BL/6J mice and in δ-KO mice and their δ-WT littermates. No differences were present in baseline body temperature between the groups (Figure 4e and j; P>0.05; the data for δ-WT littermates are not shown). There was a significant main effect of GAN treatment on body temperature (F2,41=44,84; P<0.0001). GAN produced a severe hypothermia lasting over 1.5 h (P<0.0001 in all mouse lines), but as THIP did not affect body temperature (Figure 4e and j; P>0.05), it is reasonable to conclude that GAN-induced hypothermia was unlikely the cause of aversive conditioning.
DISCUSSION
We found here that a single administration of the neurosteroid agonist GAN induced a dose-dependent glutamate receptor neuroplasticity of VTA DA neurons. We showed that GAN induced the long-lasting potentiation of VTA DA neurons at least in part through insertion of new GluA2 subunit-lacking AMPA receptors. This neuroplasticity effect was most likely induced by increased GABAA receptor-mediated tonic inhibition of the local GABAergic interneurons. The increased tonic inhibition could be acutely seen in the presence of GAN, which is known to be devoid of action on nuclear receptors. Interestingly, the neuroplasticity effect was dose dependently correlated with aversive rather than rewarding place conditioning. Finally, the conditioning effects of GAN were dependent on the δ-subunit-containing GABAA receptors, as mice lacking this subunit showed no evidence of place conditioning using the protocol that produced aversion in δ-WT mice.
GAN-Induced Glutamate Receptor Plasticity
The general pattern of our present results with GAN is consistent with our findings on modulation of VTA DA neurons by THIP (Vashchinkina et al, 2012). Interestingly, although being rapid in action and quickly metabolized (Nohria and Giller, 2007), one injection of GAN induced neuroplasticity (both increased AMPA/NMDA ratios and increased rectification of AMPA responses) in DA neurons for at least 6 days. In addition, THIP induces increased AMPA/NMDA ratios in VTA DA neurons for at least 6 days (Vashchinkina et al, 2012), whereas diazepam, preferentially acting on synaptic receptors, does it for only 3 days (Heikkinen et al, 2009). Further experiments are needed to test whether endogenous neurosteroids might serve as a new class of endogenous agents that can influence glutamate neuroplasticity in the VTA (Luscher and Malenka, 2011).
In this study, we used the Th-EGFP mouse line to identify DA neurons. Several groups have reported alterations of DA transmission in certain BAC lines (Bagetta et al, 2012; Kramer et al, 2011); therefore, we measured brain tissue DA to ascertain whether our mouse model was normal or deficient in this respect. Importantly, we found no differences in the levels of DA or its metabolites in the caudate-putamen/nucleus accumbens region or the prefrontal cortex between the Th-EGFP mice and their littermates without the transgene.
Tonic Inhibition in the VTA
First, we ascertained whether tonic inhibition is cell-type specific in the VTA, as has been shown to be the case in the hippocampus, amygdala, and thalamic nuclei (Marowsky et al, 2012; Semyanov et al, 2003). Importantly, we used two mouse lines, GAD67-GFP knockin mice and Th-EGFP BAC transgenic mice (Gong et al, 2003; Tamamaki et al, 2003), which helped us to identify the VTA GABA and DA neurons, respectively, independently of their electrophysiological characteristics (Margolis et al, 2012). Despite expression of tonic inhibition in both VTA DA and GABA neurons in the presence 500 nM GABA (in the range of physiological extracellular GABA concentration (Murphy and Maidment, 1999)), we believe that tonic inhibition is more effective in VTA GABA interneurons as these neurons have a higher input resistance than DA neurons, allowing the same charge transfer to more effectively change their membrane potential (Margolis et al, 2012). We observed tonic currents with amplitudes between 10 and 30 pA, in line with studies in various brain areas (Marowsky et al, 2012; Stell et al, 2003). Both cell types exhibited GABAergic inhibitory activity that varied in amplitude and event frequency, and some recorded cells expressed no tonic current. These data are in agreement with the idea that VTA neurons belong to different subtypes and receive different innervation (Margolis et al, 2012), showing marked heterogeneity within the DA and GABA neuronal populations.
Local application of GAN directly into the brain slice resulted in a fast and selective increase in tonic inhibition of VTA GABA cells. The range of effective neurosteroid concentrations with respect to IPSC event prolongation has been shown to vary between brain areas and cell types (for review, see Lambert et al (2003)). Our analysis of sIPSC events revealed no clear GAN effects on synaptic GABAA receptors, with only a trend for increased decay kinetics. Therefore, a moderate 500 nM concentration of GAN appeared to activate only extrasynaptic GABAA receptors mediating tonic inhibition.
We also show that applying 500 nM GAN to midbrain slices caused significantly increased AMPA/NMDA ratios in VTA DA neurons within 4–6 h. A similar time window for development of cocaine-induced plasticity in VTA has been observed earlier (Luscher and Malenka, 2011). Thus, GAN acts locally within the VTA to induce neuroplasticity in DA neurons, but we cannot exclude the possibility that GAN also acts on other brain regions and indirectly induces VTA DA neuron plasticity.
Consistent with in vitro studies described above, GAN 30 mg/kg demonstrated no effect on glutamate neuroplasticity of the VTA DA neurons in δ-KO mice. Notably, we observed similar values in AMPA/NMDA ratio in experiments with δ-WT and Th-EGFP where identification of DA neurons was based on different approaches: presence of large Ih current and EGFP marker, respectively. Given that the large Ih current is thought to be a marker for a subpopulation of VTA DA neurons (Lammel et al, 2011), it seems unlikely that GAN selectively activates particular subpopulation of VTA DA neurons.
Failure to Induce a CPP
Drug-induced CPP behavior is strongly related to DA neuron activity (Luscher and Malenka, 2011). However, changes in the DA neuron activity do not necessarily produce a response in DA release, and it is also possible that different mesolimbic VTA DA neuron populations separately mediate rewarding and aversive behaviors (Lammel et al, 2011). Indeed, repeated GAN administrations in an unbiased place-conditioning paradigm resulted in aversion rather than preference for conditioned floors in C57BL/6J mice, with which we have previously seen significant morphine- and ethanol-induced conditioned preference (Aitta-aho et al, 2012; Nuutinen et al, 2010). Further, we have found that THIP induces prolonged conditioned aversion in mice and that it fails to sustain reinforcement behavior in baboons, in contrast to benzodiazepines (Vashchinkina et al, 2012).
The behavioral effect that we observed in GAN-conditioning tests may have resulted from an effect on DA neuron activity, or on some other, presently unknown, neuronal pathway. Earlier place-conditioning work on allopregnanolone has suggested dose-dependent CPP, aversion, or no place conditioning in mice (Beauchamp et al, 2000; Finn et al, 1997; Tzschentke, 2007). Contradictory results on neurosteroid-induced conditioning could be because of procedural differences, different doses used, drug delivery methods (i.p. vs intracranial), and animal species and their age. It is noteworthy that all of the above studies used endogenous neurosteroids, whereas we used a synthetic neurosteroid that lacks nuclear steroid hormonal activity (Carter et al, 1997).
Conditioning in δ-KO Mice
Consistent with our electrophysiological results on glutamate neuroplasticity, GAN at the lower dose demonstrated no effect on behavior, whereas the higher dose produced an effect. Using the higher dose of GAN, we found that δ-WT mice showed similar conditioned aversion as C57BL/6J mice, whereas no significant expression of place conditioning was observed in δ-KO mice. There was, however, a trend toward CPP in knockout mice. The higher dose of GAN apparently induced conditioning by the action on δ-subunit-containing GABAA receptors.
Several other GAN-induced effects in δ-KO mice were somewhat puzzling but suggested targets other than the δ-GABAA receptors. First, locomotor activity was similarly disrupted by GAN in both δ-WT and δ-KO genotypes. The cerebellum and striatum expressing a high density of δ-subunits are strongly involved in motor control as reviewed in Uusi-Oukari and Korpi (2010). In both of these regions, compensatory GABAA receptor changes are known to take place in the δ-KO mice (Korpi et al, 2002; Peng et al, 2002), thus preserving the main molecular targets for the neurosteroids (Hosie et al, 2006) also in the absence of the δ-subunit. Second, similar to locomotion, both genotypes displayed identical hypothermic response to GAN. On the other hand, a closer examination of behavior in the open field of the cage during the first conditioning session revealed an absence of anxiolytic-like effects by GAN in δ-KO mice (see Figure 4g inset), as compared with δ-WT littermates, in keeping with the original study on δ-KO mice (Mihalek et al, 1999). This difference should not have produced the GAN-induced aversion in the δ-WT mice, as, if anything, an anxiolytic effect is associated with place preference (File, 1986). Finally, it is possible that the δ-subunit deletion somehow affected learning associated with conditioning. However, pharmacological or genetic manipulations reducing GABAergic inhibition often enhance synaptic plasticity and learning (Introini-Collison et al, 1994), and genetic deletion of the δ-subunit reduces inhibition in the hippocampus (Spigelman et al, 2003), a well-known area contributing especially to the formation of new spatial memories. Indeed, δ-KOs have demonstrated enhanced trace fear conditioning compared with wild-type animals (Wiltgen et al, 2005). Thus, defective neurosteroid-induced conditioning in the δ-KO mice is unlikely to be due to impaired learning. This defect may at least partly be linked to impaired neuroplasticity of the VTA DA pathway in these animals, even if there are GABAA receptor compensations also in the DAergic areas, such as the nucleus accumbens (Korpi et al, 2002; Peng et al, 2002).
CONCLUSIONS
The neurosteroid agonist GAN ex vivo induced glutamate receptor neuroplasticity in VTA DA neurons via increased tonic inhibition of VTA GABA neurons in young mice. Behaviorally, in adult mice, GAN produced CPA, which was not present in GABAA receptor δ-subunit-deficient mice. GAN has been tested in clinical trials (clinicaltrials.gov) and is well tolerated in humans (Nohria and Giller, 2007). With the present interesting neuropsychopharmacological profile of action in mind, GAN should be tested for its possible antiaddiction efficacy in humans, especially as preclinical data already exist on a dose-dependent reduction in alcohol drinking (Ramaker et al, 2012).
FUNDING AND DISCLOSURE
This work was supported by the Academy of Finland (ERK), the Sigrid Juselius Foundation (ERK), the Finnish Foundation for Alcohol Studies (EV, OV), and the Finnish Graduate School of Neuroscience (EV). The authors declare no conflict of interest.
Acknowledgments
We thank Holger Rabe and Andrei Timofeev for setting up the local drug delivery system, Juha Partanen and Yuchio Yanagawa for providing the GAD67-GFP mouse line and instructions for genotyping, and Heidi Pehkonen for skillful technical aid.
References
- Aitta-aho T, Moykkynen TP, Panhelainen AE, Vekovischeva OY, Backstrom P, Korpi ER. Importance of GluA1 subunit-containing AMPA glutamate receptors for morphine state-dependency. PLoS One. 2012;7:e38325. doi: 10.1371/journal.pone.0038325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Zlebnik NE, Carroll ME. Differential effects of allopregnanolone on the escalation of cocaine self-administration and sucrose intake in female rats. Psychopharmacology (Berl) 2010;212:419–429. doi: 10.1007/s00213-010-1968-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backstrom T, Haage D, Lofgren M, Johansson IM, Stromberg J, Nyberg S, et al. Paradoxical effects of GABA-A modulators may explain sex steroid induced negative mood symptoms in some persons. Neuroscience. 2011;191:46–54. doi: 10.1016/j.neuroscience.2011.03.061. [DOI] [PubMed] [Google Scholar]
- Bagetta V, Sgobio C, Pendolino V, Del Papa G, Tozzi A, Ghiglieri V, et al. Rebalance of striatal NMDA/AMPA receptor ratio underlies the reduced emergence of dyskinesia during D2-like dopamine agonist treatment in experimental Parkinson's disease. J Neurosci. 2012;32:17921–17931. doi: 10.1523/JNEUROSCI.2664-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MH, Ormerod BK, Jhamandas K, Boegman RJ, Beninger RJ. Neurosteroids and reward: allopregnanolone produces a conditioned place aversion in rats. Pharmacol Biochem Behav. 2000;67:29–35. doi: 10.1016/s0091-3057(00)00299-9. [DOI] [PubMed] [Google Scholar]
- Carter RB, Wood PL, Wieland S, Hawkinson JE, Belelli D, Lambert JJ, et al. Characterization of the anticonvulsant properties of ganaxolone (CCD 1042; 3α-hydroxy-3β-methyl-5α-pregnan-20-one), a selective, high-affinity, steroid modulator of the gamma-aminobutyric acidA receptor. J Pharmacol Exp Ther. 1997;280:1284–1295. [PubMed] [Google Scholar]
- Cunningham CL, Niehus JS. Drug-induced hypothermia and conditioned place aversion. Behav Neurosci. 1993;107:468–479. doi: 10.1037//0735-7044.107.3.468. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nat Protoc. 2006;1:1662–1670. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- den Hollander B, Rozov S, Linden AM, Uusi-Oukari M, Ojanpera I, Korpi ER. Long-term cognitive and neurochemical effects of ‘bath salt' designer drugs methylone and mephedrone. Pharmacol Biochem Behav. 2013;103:501–509. doi: 10.1016/j.pbb.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Fancsik A, Linn DM, Tasker JG. Neurosteroid modulation of GABA IPSCs is phosphorylation dependent. J Neurosci. 2000;20:3067–3075. doi: 10.1523/JNEUROSCI.20-09-03067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE. Aversive and appetitive properties of anxiogenic and anxiolytic agents. Behav Brain Res. 1986;21:189–194. doi: 10.1016/0166-4328(86)90236-6. [DOI] [PubMed] [Google Scholar]
- Finn DA, Phillips TJ, Okorn DM, Chester JA, Cunningham CL. Rewarding effect of the neuroactive steroid 3α-hydroxy-5α-pregnan-20-one in mice. Pharmacol Biochem Behav. 1997;56:261–264. doi: 10.1016/s0091-3057(96)00218-3. [DOI] [PubMed] [Google Scholar]
- Gee KW, McCauley LD, Lan NC. A putative receptor for neurosteroids on the GABAA receptor complex: the pharmacological properties and therapeutic potential of epalons. Crit Rev Neurobiol. 1995;9:207–227. [PubMed] [Google Scholar]
- Glykys J, Mody I. The main source of ambient GABA responsible for tonic inhibition in the mouse hippocampus. J Physiol. 2007;582:1163–1178. doi: 10.1113/jphysiol.2007.134460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Mann EO, Mody I. Which GABAA receptor subunits are necessary for tonic inhibition in the hippocampus. J Neurosci. 2008;28:1421–1426. doi: 10.1523/JNEUROSCI.4751-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Heikkinen AE, Moykkynen TP, Korpi ER. Long-lasting modulation of glutamatergic transmission in VTA dopamine neurons after a single dose of benzodiazepine agonists. Neuropsychopharmacology. 2009;34:290–298. doi: 10.1038/npp.2008.89. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Introini-Collison IB, Castellano C, McGaugh JL. Interaction of GABAergic and β-noradrenergic drugs in the regulation of memory storage. Behav Neural Biol. 1994;1:150–155. doi: 10.1016/s0163-1047(05)80068-8. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, Eberhardt H. Modulation of A10 dopamine neurons by γ-aminobutyric acid agonists. J Pharmacol Exp Ther. 1990;253:858–866. [PubMed] [Google Scholar]
- Korpi ER, Mihalek RM, Sinkkonen ST, Hauer B, Hevers W, Homanics GE, et al. Altered receptor subtypes in the forebrain of GABAA receptor δ subunit-deficient mice: recruitment of γ2 subunits. Neuroscience. 2002;109:7337–7343. doi: 10.1016/s0306-4522(01)00527-9. [DOI] [PubMed] [Google Scholar]
- Kramer PF, Christensen CH, Hazelwood LA, Dobi A, Bock R, Sibley DR, et al. Dopamine D2 receptor overexpression alters behavior and physiology in Drd2-EGFP mice. J Neurosci. 2011;31:126–132. doi: 10.1523/JNEUROSCI.4287-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Peden DR, Vardy AW, Peters JA. Neurosteroid modulation of GABAA receptors. Prog Neurobiol. 2003;71:67–80. doi: 10.1016/j.pneurobio.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Cooper MA, Simmons RD, Weir CJ, Belelli D. Neurosteroids: endogenous allosteric modulators of GABAA receptors. Psychoneuroendocrinology. 2009;34 (Suppl 1:S48–S58. doi: 10.1016/j.psyneuen.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–862. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Toy B, Himmels P, Morales M, Fields HL. Identification of rat ventral tegmental area GABAergic neurons. PLoS ONE. 2012;7:e42365. doi: 10.1371/journal.pone.0042365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marowsky A, Rudolph U, Fritschy JM, Arand M. Tonic inhibition in principal cells of the amygdala: a central role for α3 subunit-containing GABAA receptors. J Neurosci. 2012;32:8611–8619. doi: 10.1523/JNEUROSCI.4404-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior CL, Allen PM. Interaction of pregnanolone and pregnenolone sulfate with ethanol and pentobarbital. Pharmacol Biochem Behav. 1992;42:605–611. doi: 10.1016/0091-3057(92)90005-z. [DOI] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, et al. Attenuated sensitivity to neuroactive steroids in gamma-aminobutyrate type A receptor δ subunit knockout mice. Proc Natl Acad Sci USA. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy NP, Maidment NT. Orphanin FQ/nociceptin modulation of mesolimbic dopamine transmission determined by microdialysis. J Neurochem. 1999;73:179–186. doi: 10.1046/j.1471-4159.1999.0730179.x. [DOI] [PubMed] [Google Scholar]
- Nohria V, Giller E. Ganaxolone. Neurotherapeutics. 2007;4:102–105. doi: 10.1016/j.nurt.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuutinen S, Karlstedt K, Aitta-Aho T, Korpi ER, Panula P. Histamine and H3 receptor-dependent mechanisms regulate ethanol stimulation and conditioned place preference in mice. Psychopharmacology (Berl) 2010;208:75–86. doi: 10.1007/s00213-009-1710-5. [DOI] [PubMed] [Google Scholar]
- Peng Z, Hauer B, Mihalek RM, Homanics GE, Sieghart W, Olsen RW, et al. GABAA receptor changes in δ subunit-deficient mice: altered expression of α4 and γ2 subunits in the forebrain. J Comp Neurol. 2002;446:179–197. doi: 10.1002/cne.10210. [DOI] [PubMed] [Google Scholar]
- Ramaker MJ, Strong MN, Ford MM, Finn DA. Effect of ganaxolone and THIP on operant and limited-access ethanol self-administration. Neuropharmacology. 2012;63:555–564. doi: 10.1016/j.neuropharm.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci. 2003;6:484–490. doi: 10.1038/nn1043. [DOI] [PubMed] [Google Scholar]
- Sinnott RS, Phillips TJ, Finn DA. Alteration of voluntary ethanol and saccharin consumption by the neurosteroid allopregnanolone in mice. Psychopharmacology (Berl) 2002;162:438–447. doi: 10.1007/s00213-002-1123-1. [DOI] [PubMed] [Google Scholar]
- Spigelman I, Li Z, Liang J, Cagetti E, Samzadeh S, Mihalek RM, et al. Reduced inhibition and sensitivity to neurosteroids in hippocampus of mice lacking the GABAA receptor δ subunit. J Neurophysiol. 2003;90:903–910. doi: 10.1152/jn.01022.2002. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc Natl Acad Sci USA. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storustovu SI, Ebert B. Pharmacological characterization of agonists at δ-containing GABAA receptors: Functional selectivity for extrasynaptic receptors is dependent on the absence of γ2. J Pharmacol Exp Ther. 2006;316:1351–1359. doi: 10.1124/jpet.105.092403. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Uusi-Oukari M, Korpi ER. Regulation of GABAA receptor subunit expression by pharmacological agents. Pharmacol Rev. 2010;62:97–135. doi: 10.1124/pr.109.002063. [DOI] [PubMed] [Google Scholar]
- VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL. Neuroactive steroid 3α-hydroxy-5α-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J Neurosci. 2000;20:1982–1989. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashchinkina E, Panhelainen A, Vekovischeva OY, Aitta-aho T, Ebert B, Ator NA, et al. GABA site agonist gaboxadol induces addiction-predicting persistent changes in ventral tegmental area dopamine neurons but is not rewarding in mice or baboons. J Neurosci. 2012;32:5310–5320. doi: 10.1523/JNEUROSCI.4697-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Ferguson C, Homanics GE, Fanselow MS. Trace fear conditioning is enhanced in mice lacking the δ subunit of the GABAA receptor. Learn Mem. 2005;12:327–333. doi: 10.1101/lm.89705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski CF, Paul SM, Izumi Y, Covey DF, Mennerick S. Neurosteroids, stress and depression: potential therapeutic opportunities. Neurosci Biobehav Rev. 2013;37:109–122. doi: 10.1016/j.neubiorev.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]