FIGURE 1.
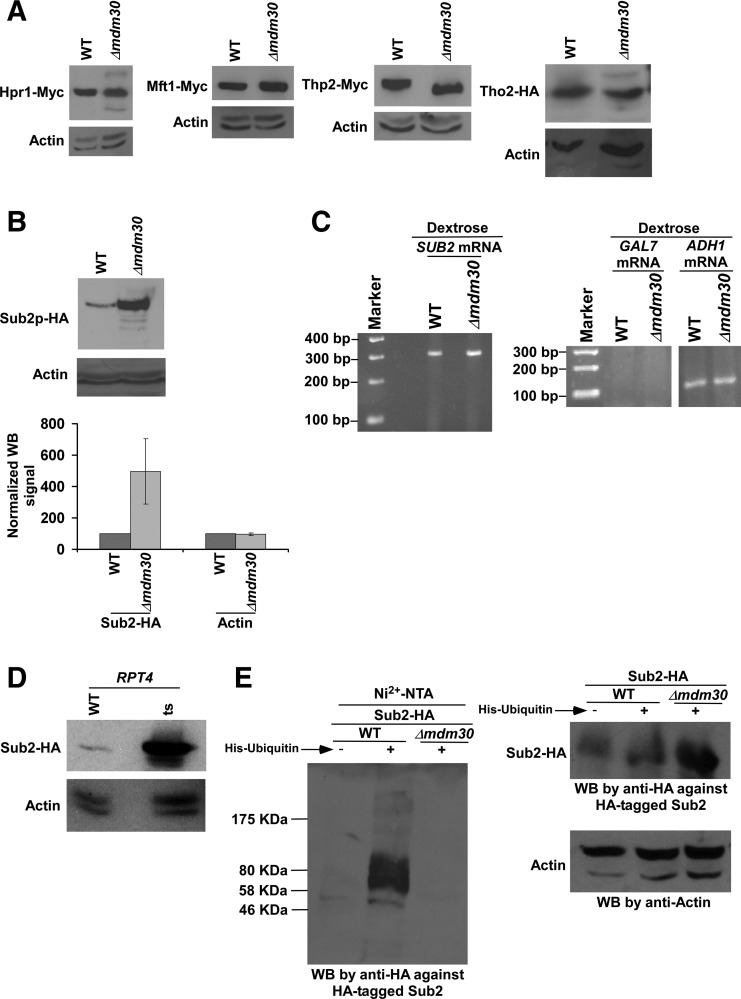
The abundance of Sub2 is enhanced in the absence of Mdm30. (A,B) Analysis of the global levels of Hpr1, Mft1, Thp2, Tho2, Sub2, and actin in the Δmdm30 and wild-type strains. Yeast strains expressing HA/Myc-tagged proteins were grown in YPD at 30°C up to an OD600 of 1.0 prior to harvesting for Western blot analysis. An anti-Myc, anti-HA, or anti-actin antibody was used in the Western blot analysis. Western blot signal of Sub2 in wild-type strain was set to 100. Western blot signal of Sub2 in the Δmdm30 strain was normalized with respect to 100. Likewise, actin levels were normalized. Normalized Western blot signals were plotted in the form of a histogram. Error bar denotes standard deviation (STDEV; Microsoft Excel 2003) from three biologically independent experiments. (WB) Western blot. (C) RT-PCR analysis of SUB2 mRNA levels in the Δmdm30 and wild-type strains using oligo-dT primer in the cDNA synthesis. Yeast strains were grown as in A. GAL7 and ADH1 mRNA levels were analyzed as controls. (D) Analysis of Sub2's abundance in the rpt4-ts and wild-type strains. Both the wild-type and ts mutant strains expressing HA-tagged Sub2 were grown in YPD at 23°C up to an OD600 of 0.85, and then switched to 39°C for 1 h before harvesting for Western blot analysis. (E) Ubiquitylation analysis of Sub2. Yeast strains expressing HA-tagged Sub2 and/or hexahistidine-tagged ubiquitin were grown in synthetic complete medium at 30°C up to an OD600 of 0.7, and then treated with CuSO4 at a final concentration of 0.1 M for 6 h. Precipitation was carried out by Ni2+-NTA agarose beads, and Western blot analysis was performed using an anti-HA antibody against HA-tagged Sub2.