Abstract
Diffuse gliomas consist of both low- and high-grade varieties, each with distinct morphological and biological features. The often extended periods of relative indolence exhibited by low-grade gliomas (LGG; WHO grade II) differ sharply from the aggressive, rapidly fatal clinical course of primary glioblastoma (GBM; WHO grade IV). Nevertheless, until recently, the molecular foundations underlying this stark biological contrast between glioma variants remained largely unknown. The discoveries of distinctive and highly recurrent genomic and epigenomic abnormalities in LGG have both informed a more accurate classification scheme and pointed to viable avenues for therapeutic development. As such, the field of neuro-oncology now seems poised to capitalize on these gains to achieve significant benefit for LGG patients. This report will briefly recount the proceedings of a workshop held in January 2013 and hosted by Accelerate Brain Cancer Cure (ABC2) on the subject of LGG. While much of the meeting covered recent insights into LGG biology, its focus remained on how best to advance the clinical management, whether by improved preclinical modeling, more effective targeted therapeutics and clinical trial design, or innovative imaging technology.
Keywords: clinical trials, genomics, low-grade glioma, personalized medicine
Diffuse gliomas of adulthood are unified by a shared propensity to widely infiltrate surrounding normal brain parenchyma, a property that effectively renders them incurable. However, the full spectrum of diffuse glioma features considerable clinical heterogeneity. More specifically, patients with glioblastoma (GBM; WHO grade IV) demonstrate overall survival times of ∼15 months,1 while those affected by low-grade (WHO grade II) astrocytomas and oligodendrogliomas frequently exhibit prolonged clinical courses lasting years or even decades.2 Low-grade gliomas (LGGs) almost invariably recur and progress to high-grade status (WHO grade III-IV). Nevertheless, their characteristically extended periods of indolence would seem to invite targeted intervention with “designer therapeutics” as an option for long-term disease control. In this way, LGGs would not simply be managed as “GBMs in waiting” and would instead drive distinct algorithms for clinical management.
Until quite recently, the systematic study of LGGs has been hindered by the somewhat subjective histopathological criteria by which specific diagnostic categories were designated, coupled with the lack of well-defined molecular drivers for the tumors themselves. Recent advances in the genomic characterization of LGGs, notably the discoveries of pathogenic mutations in IDH1, IDH2, ATRX, CIC, and FUBP1, have both clarified the molecular pathogenesis of these tumors and established robust markers for their classification.3–9 Now more than ever, the field of neuro-oncology seems poised for tangible gains in the development and refinement of customized treatment strategies for LGG. In light of these significant developments, a small group of neuro-oncologists, neurosurgeons, neuropathologists, radiation oncologists, and basic scientists gathered in Sausalito,California, in January 2013 for a meeting sponsored by Accelerate Brain Cancer Cure (ABC2) in collaboration with the University of California, San Francisco, and the University of Texas MD Anderson Cancer Center. The primary aim of the 2-day conference, entitled the “Low Grade Glioma Research Workshop,” was to assess the evolving landscape of basic and clinical research on LGGs with an eye towards optimizing preclinical testing and therapeutic trials moving forward. This report will describe the essentials of the various presentations and discuss some of the central themes emerging from the workshop that are likely to guide forthcoming investigative efforts.
Optimizing Current Treatment Modalities for Low-grade Giloma
While the clinical management of GBM has become highly standardized during the past decade, well-defined protocols for the treatment of LGG are lacking. Of note, considerable uncertainty remains regarding the most appropriate use of surgery, ionizing radiation (IR), and cytotoxic chemotherapy, all of which are mainstays in the treatment of GBM. Several talks at the LGG Research Workshop spoke directly to open questions such as these. Mitch Berger (University of California, San Francisco) described recent work demonstrating that the extent of surgical resection can strongly impact LGG patient outcome.10 In particular, gross total resections resulted in extended progression-free survival (PFS), not infrequently exceeding 10 years. His findings support more aggressive resection of LGGs upfront, employing detailed cortical mapping as needed to facilitate thorough dissection in eloquent brain regions.
Determining the optimal clinical context for radiotherapy in the management of LGG represents an additional challenge for the field. In her talk on this subject, Daphne Haas-Kogan (University of California, San Francisco) highlighted data from EORTC (European Organisation for Research and Treatment of Cancer) 22845 indicating that early postoperative radiotherapy delays LGG progression but does not appear to impact overall survival (see Table 1 for information on clinical trials disussed in this report).11 Moreover, results from EORTC 22844 showed no evidence of increased efficacy for higher doses of IR compared with lower doses.12 She also discussed which patients should receive IR in the postoperative setting, arguing that indications for treatment include progressive disease after observation, significant, new or worsening neurological symptoms, age >40 years, and poorly controlled seizures. That being said, she described data from RTOG (Radiation Therapy Oncology Group) 9802 and 0925 indicating that simple observation may be an acceptable, if not necessarily superior, course of action for some LGGs.13
Riccardo Soffietti (University Hospital, Turin) reviewed a number of issues surrounding the usage of chemotherapy in LGG patients. He cited early data from RTOG 9802 suggesting that procarbazine, N-(2-chloroethyl)-N′-cyclohexyl-N-nitrosourea, and vincristine (PCV) chemotherapy, combined with ionizing radiation, thus far improves PFS in LGG patients but not overall survival.13 He also discussed ongoing EORTC and RTOG trials, which should provide further clarification as to whether temozolomide alone or concomitant radiotherapy and temozolomide represent superior upfront treatment regimens to radiotherapy alone. Moreover, he argued that chemotherapy alone may itself play an important role in symptomatic reduction for LGG, particularly in the setting of seizures, while temporarily sparing patients radiotherapy to the CNS and its associated risk of cognitive deficits.14,15
Multiple speakers stressed the importance of radiographic assessment, particularly in the determination of malignant progression and therapeutic response, and the considerable challenges posed by LGGs in this regard. Dan Cahill (Massachusetts General Hospital) spoke directly to this issue, emphasizing that for LGGs in particular, infiltrative growth patterns, pseudoprogression, and difficulties distinguishing symptoms arising from tumor growth from those arising from treatment side effects complicate the integration of radiographic findings with clinical status. Moreover, he argued that standard paradigms correlating contrast enhancement with malignant progression may be inadequate for the effective assessment of LGGs, especially the large majority harboring IDH mutations (see below) whose fundamental biology differs sharply from that of primary GBM. Finally, he cited 2 studies whose findings indicated that, as a consequence of intratumoral heterogeneity, surgical undersampling can result in diagnoses not reflective of the tumor as a whole,16,17 and suggested that focused magnetic resonance spectroscopy could greatly facilitate the selection of more appropriate biopsy targets.
Emerging Molecular Foundations of Low-grade Glioma
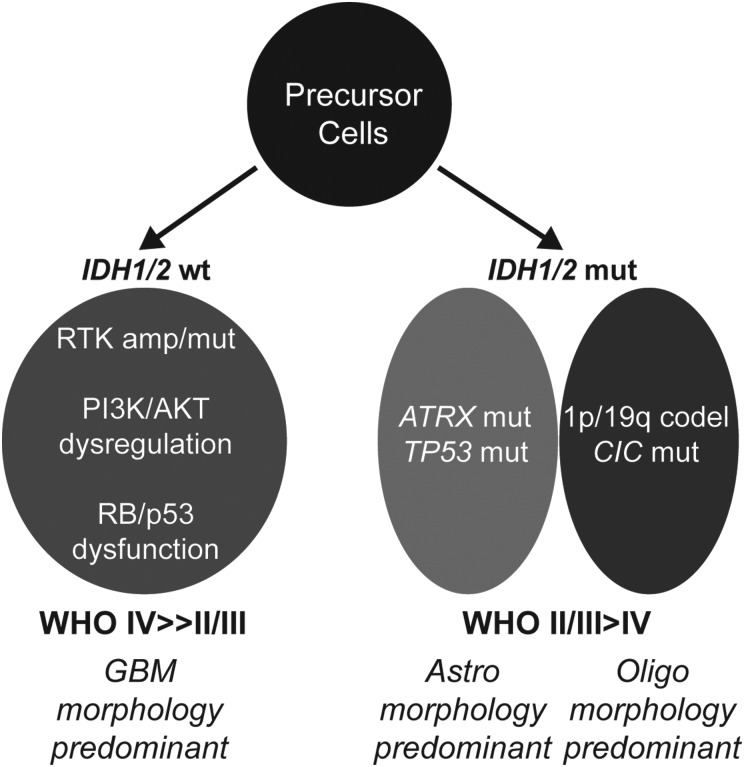
As alluded to above, genomic profiling efforts have revealed a defined set of molecular abnormalities occurring at high rates in LGG that by contrast are largely absent in primary GBM. Indeed, recently discovered somatic mutations in IDH1/2, ATRX, CIC, and FUBP1 have dramatically altered conceptions of low-grade gliomagenesis while also informing more robust diagnostic classification schemes (Fig. 1). A number of talks at the LGG Research Workshop covered recent work identifying and characterizing these genomic events. Mutations in IDH1 and its homologue IDH2 have been shown to occur in 70%–90% of LGGs and the higher-grade tumors into which they evolve.7,8 Through production of the oncometabolite R(-)-2-hydroxy-glutarate (2HG), IDH mutations appear to dysregulate cellular epigenomic landscapes, hamper normal differentiation processes, and impede the tumor-suppressive functions of HIF1α, all of which likely contribute to the initiation of gliomagenesis.18–22 Ken Aldape (MD Anderson Cancer Center) described a series of studies examining how IDH mutational status correlated with histopathological features and clinical outcome in both WHO grade II and WHO grade III diffuse gliomas. Using a cohort of 559 tumors consisting of both astrocytic and oligodendroglial glioma subtypes, his group found that IDH mutational status clearly outperformed standard WHO histopathological grading in terms of prognostic stratification, with mutant tumors exhibiting better prognosis, which was consistent with previous reports.8,23–25 Moreover, histopathological features, whether standard WHO grading metrics or measures of proliferative activity (eg, pHH3 immunohistochemistry), demonstrated little if any association with clinical outcome for IDH-mutant tumors, which also echoed findings from earlier work.23 By contrast, proliferative activity was highly predictive in IDH-wt tumors designating an aggressive “GBM-like” subset.
Fig. 1.
Molecular subclasses of diffuse glioma. IDH-wt tumors frequently exhibit receptor tyrosine kinase (RTK) amplification and/or mutation and genomic dysregulation of PI3K/AKT, RB, and p53 pathways. IDH-mutant diffuse gliomas harbor either ATRX and TP53 mutation or 1p/19q codeletion (frequently in combination with CIC mutation) in a mutually exclusive manner. Histopathological trends regarding WHO grade and morphology are also shown. Abbreviations: Astro, astrocytic; Oligo, oligodendroglial.
Table 1.
Clinical trials discussed in this meeting report
| Title/Description | Status | NCI Registration # | Additional ID # |
|---|---|---|---|
| A Phase II Study of Observation in Favorable Low-Grade Glioma and a Phase II Study of Radiation With or Without PCV Chemotherapy in Unfavorable Low-grade Glioma | Closed | NCT00003375 | RTOG 9802 |
| Natural History of Postoperative Cognitive Function, Quality of Life, and Seizure Control in Patients With Supratentorial Low-Risk Grade II Glioma | Active | NCT01417507 | RTOG 0925 |
| Phase III Randomized Comparison of Early vs No or Late Radiotherapy in Adult Patients with Grade I/II Supratentorial Astrocytomas and Oligodendrogliomas | Closed | EORTC 22845 | |
| Phase III Randomized Comparison of Two Radiotherapy Doses Following Surgery in Adults with Grade I/II Supratentorial Astrocytoma and Oligodendroglioma | Closed | EORTC 22844 | |
| Radiation Therapy or Temozolomide in Treating Patients With Gliomas | Closed | NCT00182819 | EORTC 22033-26033 |
| A Phase II Study of BKM 120 for Patients With Recurrent Glioblastoma and Activated PI3K Pathway | Active | NCT01339052 | |
| Phase II Trial of RAD001 in Patients With Recurrent Low Grade Glioma | Active | NCT00823459 |
IDH-mutant LGGs harbor additional highly recurrent molecular abnormalities that correlate with tumor morphology. For instance, it has long been appreciated that coincident loss of chromosomes 1p and 19q by way of a unique translocation event—t(1;19)(q10;p10)—is highly enriched in oligodendroglioma26,27 and that this genomic abnormality may silence crucial, disease-relevant tumor suppressors. This latter conjecture implies that 1p/19q codeleted gliomas may also harbor inactivating mutations in putative tumor suppressors on undeleted copies of chromosomes 1p and 19q. Stephen Yip (Vancouver General Hospital) and Chetan Bettegowda (Johns Hopkins Medical Institutions) each presented results from recent deep sequencing studies exploring this hypothesis.3,9 In both cases, mutations in the CIC gene on chromosome 19q and, to a lesser extent the FUBP1 gene on chromosome 1p, were found to be highly enriched in 1p/19q codeleted LGGs. Dr. Yip also described work identifying promoter hypermethylation at the NHE-1 locus on chromosome 1p as a frequent occurrence in this tumor subset.28 While the precise functional roles of CIC, FUBP1, and NHE-1 in normal cell biology, as well as the consequences of their deficiency on gliomagenic transformation, are unclear, intriguing associations with oncogenic signaling networks and gene regulation provide exciting avenues for future investigations.
Dr. Bettegowda also reviewed several recent reports describing loss-of-function ATRX mutations in the majority of IDH-mutant, 1p/19q-intact LGGs, predominantly those exhibiting astrocytic morphology.4–6 ATRX is involved in the maintenance and remodeling of chromatin, particularly at heterochromatic regions like telomeres.29 ATRX deficiency appears to induce pathological telomere maintenance via so-called “alternative lengthening of telomeres” (ALT), a potential molecular mechanism enabling cellular immortalization.30 As an aside, more recent data have demonstrated that activating promoter mutations in TERT, the core enzymatic component of telomerase, are mutually exclusive with ATRX mutations in IDH-mutant LGGs (occurring primarily in the context of 1p/19q codeletion), further supporting the notion that pathological telomere maintenance is required for the pathogenesis of these tumors.31 Whether ALT or any other physiological sequelae of ATRX mutation mediates low-grade gliomagenesis remains to be established. Regardless, the mutual exclusivity that exists between ATRX and CIC/FUBP1 mutations in IDH-mutant LGGs forms the foundation of an improved classification scheme (Fig. 1). Emphasizing this, Dr. Bettegowda presented survival data showing that IDH-mutant, CIC/FUBP1-mutant gliomas performed significantly better than IDH-mutant, ATRX-mutant gliomas, with both groups demonstrating favorable prognosis relative to their IDH-wt counterparts.4
The Cancer Genome Atlas (TCGA) has recently embarked on a large-scale, multidimensional, molecular analysis of LGG. Dan Brat (Emory University), who heads the effort, and Sofie Salama (University of California, Santa Cruz) provided a general overview of the project and described preliminary data emerging from the various profiling pipelines. While much work remains to be done, early findings have confirmed the pervasive occurrence and coexistent patterns of IDH1/2, ATRX, CIC, and FUBP1 mutations. Moreover, IDH mutation associates with a CpG island hypermethylator phenotype, as expected, with IDH-wt LGGs standing out as a distinctly hypomethylated subgroup whose genomic profile strikingly resembles that of primary GBM (eg, common copy number alterations in chromosomes 7 and 10). Gene expression clusters that correlate with genomic and epigenomic signatures are also emerging. Further results from TCGA will be highly anticipated by the neuro-oncology community, with the first report expected in the spring/summer of 2014.
Preclinical Modeling of Low-grade Glioma
As research into LGG continues to reveal promising therapeutic strategies, the need for robust preclinical models will only continue to grow. And while many such experimental systems, both in vitro and in vivo, currently exist for GBM, their specific development for the study of LGG has lagged far behind. This challenge was discussed explicitly at the LGG Research Workshop. C Ryan Miller (University of North Carolina, Chapel Hill) discussed a series of genetically engineered mouse models, recently produced by his lab, that appear to recapitulate the core biological features of LGG with periods of relative indolence followed by high-grade transformation; this despite their derivation from molecular abnormalities classically associated with GBM, namely retinoblastoma (RB) dysfunction, PTEN loss, and RAS/MAPK pathway activation. He found that the gene expression patterns of the various models segregated not only with specific molecular drivers of tumorigenesis but also tended to reflect cell of origin. He observed that the majority of WHO grade II tumors emerging from his models involved the olfactory bulb, the final migratory destination for subventricular zone (SVZ) precursors; this observation was consistent with published work implicating neuroglial progenitors in the SVZ as potential cells of origin for LGG.23,32 In all cases, high-grade transformation occurred stochastically but frequently in association with recurrent DNA copy number abnormalities involving Met on murine chromosome 6.
Jason Huse (Memorial Sloan-Kettering Cancer Center) argued that optimal preclinical models for LGG should be grounded in the now-established genomic events driving disease-specific tumorigenesis. While acknowledging that such experimental systems have been difficult to generate, particularly in genetically engineered mice, he proposed that a more faithful recapitulation of the precise molecular and cellular context of low-grade gliomagenesis would be essential moving forward, if only to definitively test the pathogenic sufficiency of IDH1/2, ATRX, CIC, FUBP1 mutations. Reviewing recent literature, he stressed the importance of targeting transformative events to SVZ neuroglial progenitors, the likely cells of origin for LGG. He also presented in vitro systems based on transformed human astrocytes and cultured murine neural stem cells, which appear to effectively model many of the core biological properties characterizing IDH-mutant gliomagenesis.
Clinical Trials and Novel Therapeutics
Multiple sessions at the LGG Research Workshop were devoted to the subject of clinical trials for LGG, with a focus on promising therapeutic agents and more effective study design. The phosphatidylinositol-3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) signaling network has been repeatedly implicated in glioma biology over the past decade.33,34 However, a defined role for the inhibition of this pathway in the treatment of LGG remains to be established. Patrick Wen (Dana Farber Cancer Institute) addressed this and other issues in his talk covering targeted therapies for glioma. First, he described an ongoing trial of BKM120, an oral pan-class I PI3K inhibitor, in patients with recurrent GBM in which he highlighted design elements, such as specific molecular enrollment criteria, that will likely improve study sensitivity. He also discussed the promise of targeting the RAS/mitogen activated protein kinase (MAPK) signaling network in selected pediatric and adult low-grade glioma variants, such as ganglioglioma and pleomorphic xanthoastrocytoma, which have recently been shown to frequently and, in some cases invariably, harbor molecular abnormalities in BRAF, a central pathway constituent.35,36 Finally, he discussed how to best construct LGG trials, arguing that radiographic response and/or PFS each represent superior endpoints to overall survival in this specific disease context, given the extended clinical course exhibited by many patients. In doing so, he reviewed the recently published Response Assessment in Neuro-Oncology (RANO) criteria for LGG and how they differ from those typically employed for GBM and other high-grade gliomas, notably in their incorporation of minor response criteria and considerations of patient-reported outcomes and seizure control.37
Daphne Haas-Kogan presented preliminary findings from an ongoing phase II trial of the mTOR inhibitor everolimus in recurrent LGG. In this study, patients with histopathological evidence of recurrence and/or progression were treated with oral everolimus daily followed by clinical and radiographic monitoring every 2–3 months. PI3K pathway activation was assessed immunohistochemically using a number of markers including phospho-PRAS40, phospho-S6, and PTEN. Interestingly, early results suggest increased PFS in patients whose tumors are positive for phospho-PRAS40, presumably indicating PI3K pathway activity. Should this correlation persist when the data are fully mature, it would potentially guide the management of LGG in the recurrent setting.
Much attention at the LGG Research Workshop was paid to the prospect of targeting therapies to the mutant IDH protein that essentially defines LGG pathogenesis. Kate Yen (Agios Pharmaceuticals) detailed the results of recent preclinical work on AGI-5198, a selective inhibitor of mutant IDH1.38 At nanomolar levels, AGI-5198 appears to abrogate 2HG production, re-establish normal differentiation processes in IDH1-mutant cells, and impair the growth of IDH1-mutant xenografts in mice. Moreover, inhibition of mutant IDH1 partially reverts some of the epigenomic alterations induced by elevated 2HG. These promising results indicate that targeting mutant IDH may prove to be a viable treatment strategy for LGG and pave the way for clinical trials to formally address this possibility.
Tim Cloughesy (University of California, Los Angeles) then discussed clinical trial design for mutant IDH inhibitors. He raised several important questions regarding pharmacodynamic validation, patient enrollment criteria, and efficacy assessments. For instance, what patient pools (eg, newly diagnosed LGG, recurrent/treated secondary GBM, etc.) are optimally suited for mutant IDH1 inhibitor trials at the phase I and/or proof-of-concept stages? Can 2HG serve as a pharmacodynamic marker, can it be assessed noninvasively, and will its levels correlate with disease response? Finally, what constitutes the optimal endpoint for efficacy trials (eg, response, PFS, etc.), and how should such endpoints be assessed? He then presented a series of hypothetical trial structures that might effectively address these and other related issues. In doing so, he alluded to the vital importance of radiographic assessment for both pharmacodynamic and clinical endpoint determinations. Sarah Nelson (University of California, San Francisco) spoke at greater length on this subject and described recently successful efforts to detect 2HG by magnetic resonance spectroscopy.39 She also covered progress in the development of other agents for imaging metabolic markers such as hyperpolarized 13C-labeled pyruvate and α-ketoglutarate.40
Personalizing the Management of Low-grade Glioma
As therapeutic regimens for LGG evolve in the ensuing years, studying the biological behavior of these tumors in the context of therapy will become increasingly important. Addressing this subject, Joseph Costello (University of California, San Francisco) presented recent data from his group that described patterns of genomic alterations acquired by LGGs during treatment with cytotoxic chemotherapy. In a sample cohort consisting of case-matched pairs of astrocytic LGGs, each consisting of one WHO grade II or III tumor and the WHO grade IV GBM into which it evolved, he found that 6 of 8 patients receiving temozolomide each acquired more than 1 000 novel mutations prior to their tumors recurring as GBMs. By contrast, recurrences of all grades in the remaining 16 sample pairs harbored less than 75 somatic mutations each. Temozolomide-associated mutations were highly enriched in C:G > T:A transitions, consistent with their induction by alkylating agent therapy and frequently affected key amino acids in constituents of bona fide oncogenic networks (eg, RB and PI3K signaling pathways). These findings raise important questions regarding the most appropriate use of chemotherapy in LGGs and how best to therapeutically manage genomic consequences at recurrence.
Fine-tuning treatment regimens around the molecular evolution of specific tumors will require the routine employment of high-throughput genomic technology in the clinical setting. In a talk prepared by Marco Marra (British Columbia Cancer Agency), Stephen Yip reported on early experiences with clinical genomics at their institution. He described a 5- to 6-week workflow encompassing tumor biopsy, biomaterial extraction, sequencing (some combination of targeted capture sequencing, whole genome sequencing, and RNA sequencing), data analysis, therapeutic recommendations, and final reporting. Through a series of case studies, he then touched on some of the significant lessons learned during the initial implementation of this pipeline. For instance, biopsies from recurrences frequently yielded substantially different genomic profiles than their respective primary tumors, emphasizing the importance of adequate sampling for thorough analysis. Moreover, genomic alterations could be selected by specific therapeutic regimens. Finally, and perhaps most significantly, they found that genomic profiling could both inform diagnosis and alter treatment approach.
Concluding Remarks
The recent molecular characterization of LGG has provided not only a clarified framework for the conceptualization of these tumors but has also revealed pathways for the development of more effective targeted therapeutics. Both of these factors should dramatically accelerate the pace of LGG research, with significant changes in clinical management hopefully not far behind. As the process of therapeutic refinement moves forward, more effective preclinical models and optimal clinical trial design will be absolutely crucial, as will the ready availability of sophisticated genomic technology in the clinical environment. Moreover, multidisciplinary and international collaborative efforts will be critical to address the broad aspects of LGG. While these are exciting times indeed, much work remains to be done.
Supplementary Material
Funding
None declared.
Supplementary Material
Acknowledgments
We would like to graciously acknowledge the staff at Cavallo Point Lodge for hosting a wonderful event.
Conflict of interest statement. PYW sits on the advisory board and receives research support from Novartis Pharmaceuticals. KY is a full-time employee at Agios Pharmaceuticals with equity in the company. MW is the chief executive officer of Accelerate Brain Cancer Cure. Remaining authors have no conflicts of interest to disclose.
References
- 1.Wen PY, Kesari S. Malignant Gliomas in Adults. N Engl J Med. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 2.Lang FF, Gilbert MR. Diffusely infiltrative low-grade gliomas in adults. J Clin Oncol. 2006;24(8):1236–1245. doi: 10.1200/JCO.2005.05.2399. [DOI] [PubMed] [Google Scholar]
- 3.Bettegowda C, Agrawal N, Jiao Y, et al. Mutations in CIC and FUBP1 contribute to human oligodendroglioma. Science. 2011;333(6048):1453–1455. doi: 10.1126/science.1210557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiao Y, Killela PJ, Reitman ZJ, et al. Frequent ATRX, CIC, and FUBP1 mutations refine the classification of malignant gliomas. Oncotarget. 2012;3(7):709–722. doi: 10.18632/oncotarget.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kannan K, Inagaki A, Silber J, et al. Whole exome sequencing identified ATRX mutation as a key molecular determinant in lower-grade glioma. Oncotarget. 2012;3(10):1194–1203. doi: 10.18632/oncotarget.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu XY, Gerges N, Korshunov A, et al. Frequent ATRX mutations and loss of expression in adult diffuse astrocytic tumors carrying IDH1/IDH2 and TP53 mutations. Acta Neuropathol. 2012;124(5):615–625. doi: 10.1007/s00401-012-1031-3. [DOI] [PubMed] [Google Scholar]
- 7.Parsons DW, Jones S, Zhang X, et al. An Integrated Genomic Analysis of Human Glioblastoma Multiforme. Science (New York, N.Y.) 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yip S, Butterfield YS, Morozova O, et al. Concurrent CIC mutations, IDH mutations, and 1p/19q loss distinguish oligodendrogliomas from other cancers. J Pathol. 2012;226(1):7–16. doi: 10.1002/path.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26(8):1338–1345. doi: 10.1200/JCO.2007.13.9337. [DOI] [PubMed] [Google Scholar]
- 11.van den Bent MJ, Afra D, de Witte O, et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366(9490):985–990. doi: 10.1016/S0140-6736(05)67070-5. [DOI] [PubMed] [Google Scholar]
- 12.Karim AB, Maat B, Hatlevoll R, et al. A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) Study 22844. Int J Radiat Oncol Biol Phys. 1996;36(3):549–556. doi: 10.1016/s0360-3016(96)00352-5. [DOI] [PubMed] [Google Scholar]
- 13.Shaw EG, Wang M, Coons SW, et al. Randomized trial of radiation therapy plus procarbazine, lomustine, and vincristine chemotherapy for supratentorial adult low-grade glioma: initial results of RTOG 9802. J Clin Oncol. 2012;30(25):3065–3070. doi: 10.1200/JCO.2011.35.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soffietti R, Baumert BG, Bello L, et al. Guidelines on management of low-grade gliomas: report of an EFNS-EANO Task Force. Eur J Neurol. 2010;17(9):1124–1133. doi: 10.1111/j.1468-1331.2010.03151.x. [DOI] [PubMed] [Google Scholar]
- 15.Viaccoz A, Lekoubou A, Ducray F. Chemotherapy in low-grade gliomas. Curr Opin Oncol. 2012;24(6):694–701. doi: 10.1097/CCO.0b013e328357f503. [DOI] [PubMed] [Google Scholar]
- 16.Glantz MJ, Burger PC, Herndon JE, 2nd, et al. Influence of the type of surgery on the histologic diagnosis in patients with anaplastic gliomas. Neurology. 1991;41(11):1741–1744. doi: 10.1212/wnl.41.11.1741. [DOI] [PubMed] [Google Scholar]
- 17.Jackson RJ, Fuller GN, Abi-Said D, et al. Limitations of stereotactic biopsy in the initial management of gliomas. Neuro Oncol. 2001;3(3):193–200. doi: 10.1093/neuonc/3.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462(7274):739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Figueroa ME, Abdel-Wahab O, Lu C, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18(6):553–567. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koivunen P, Lee S, Duncan CG, et al. Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature. 2012;483(7390):484–488. doi: 10.1038/nature10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu C, Ward PS, Kapoor GS, et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483(7390):474–478. doi: 10.1038/nature10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turcan S, Rohle D, Goenka A, et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012;483(7390):479–483. doi: 10.1038/nature10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorovets D, Kannan K, Shen R, et al. IDH mutation and neuroglial developmental features define clinically distinct subclasses of lower grade diffuse astrocytic glioma. Clin Cancer Res. 2012;18(9):2490–2501. doi: 10.1158/1078-0432.CCR-11-2977. [DOI] [PubMed] [Google Scholar]
- 24.Hartmann C, Hentschel B, Wick W, et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 2010;120(6):707–718. doi: 10.1007/s00401-010-0781-z. [DOI] [PubMed] [Google Scholar]
- 25.Sanson M, Marie Y, Paris S, et al. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol. 2009;27(25):4150–4154. doi: 10.1200/JCO.2009.21.9832. [DOI] [PubMed] [Google Scholar]
- 26.Kraus JA, Koopmann J, Kaskel P, et al. Shared allelic losses on chromosomes 1p and 19q suggest a common origin of oligodendroglioma and oligoastrocytoma. J Neuropathol Exp Neurol. 1995;54(1):91–95. doi: 10.1097/00005072-199501000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Reifenberger G, Reifenberger J, Liu L, James CD, Wechsler W, Collins VP. Molecular genetic analysis of oligodendroglial tumors shows preferential allelic deletions on 19q and 1p. Am J Pathol. 1994;145(5):1175–1190. [PMC free article] [PubMed] [Google Scholar]
- 28.Blough MD, Al-Najjar M, Chesnelong C, et al. DNA hypermethylation and 1p Loss silence NHE-1 in oligodendroglioma. Ann Neurol. 2012;71(6):845–849. doi: 10.1002/ana.23610. [DOI] [PubMed] [Google Scholar]
- 29.Elsasser SJ, Allis CD, Lewis PW. Cancer. New epigenetic drivers of cancers. Science. 2011;331(6021):1145–1146. doi: 10.1126/science.1203280. [DOI] [PubMed] [Google Scholar]
- 30.Heaphy CM, de Wilde RF, Jiao Y, et al. Altered telomeres in tumors with ATRX and DAXX mutations. Science. 2011;333(6041):425. doi: 10.1126/science.1207313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110(15):6021–6026. doi: 10.1073/pnas.1303607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai A, Kharbanda S, Pope WB, et al. Evidence for sequenced molecular evolution of IDH1 mutant glioblastoma from a distinct cell of origin. J Clin Oncol. 2011;29(34):4482–4490. doi: 10.1200/JCO.2010.33.8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huse JT, Holland E, DeAngelis LM. Glioblastoma: molecular analysis and clinical implications. Annu Rev Med. 2013;64:59–70. doi: 10.1146/annurev-med-100711-143028. [DOI] [PubMed] [Google Scholar]
- 34.Huse JT, Holland EC. Targeting brain cancer: advances in the molecular pathology of malignant glioma and medulloblastoma. Nat Rev Cancer. 2010;10(5):319–331. doi: 10.1038/nrc2818. [DOI] [PubMed] [Google Scholar]
- 35.Dias-Santagata D, Lam Q, Vernovsky K, et al. BRAF V600E mutations are common in pleomorphic xanthoastrocytoma: diagnostic and therapeutic implications. PloS One. 2011;6(3):e17948. doi: 10.1371/journal.pone.0017948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez FJ, Lim KS, Bowers D, Eberhart CG. Pathological and molecular advances in pediatric low-grade astrocytoma. Annu Rev Pathol. 2013;8:361–379. doi: 10.1146/annurev-pathol-020712-164009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van den Bent MJ, Wefel JS, Schiff D, et al. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011;12(6):583–593. doi: 10.1016/S1470-2045(11)70057-2. [DOI] [PubMed] [Google Scholar]
- 38.Rohle D, Popovici-Muller J, Palaskas N, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science. 2013;340(6132):626–630. doi: 10.1126/science.1236062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elkhaled A, Jalbert LE, Phillips JJ, et al. Magnetic resonance of 2-hydroxyglutarate in IDH1-mutated low-grade gliomas. Sci Transl Med. 2012;4(116):116ra115. doi: 10.1126/scitranslmed.3002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nelson SJ, Ozhinsky E, Li Y, Park I, Crane J. Strategies for rapid in vivo 1H and hyperpolarized 13C MR spectroscopic imaging. J Magn Reson. 2013;229:187–197. doi: 10.1016/j.jmr.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.