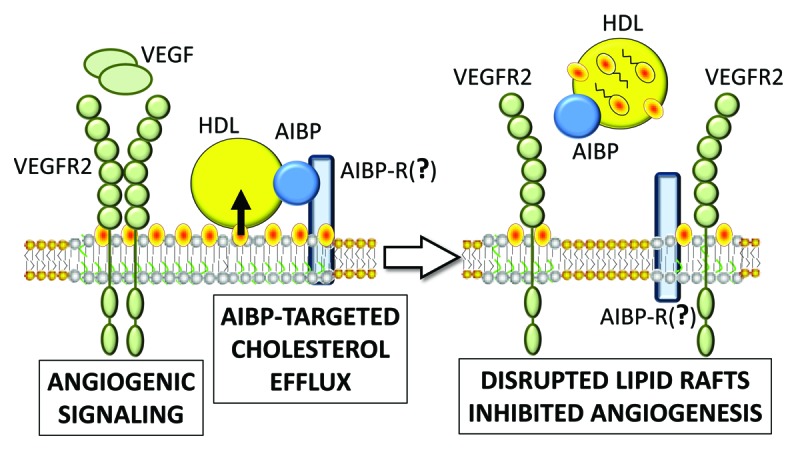
Figure 1. AIBP-targeted cholesterol efflux from EC disrupts lipid rafts and inhibits angiogenesis. VEGFR2 signaling in response to VEGF requires the receptor dimerization, which occurs in the cholesterol-rich membrane microdomains, often designated as lipid rafts. As the name implies, AIBP binds apoA-I and HDL; it also binds EC in a saturable manner, which suggests a receptor-mediated binding, but the hypothetical AIBP receptor (AIBP-R) has not yet been identified. AIBP increases the overall HDL binding to EC and at the same time reduces the affinity of HDL binding, thereby creating conditions for accelerated cholesterol efflux. Enhanced removal of cholesterol from the EC plasma membrane disrupts lipid rafts, which, in turn, inhibits VEGFR2 signaling and VEGF-induced angiogenesis. HDL transports cholesterol removed from the cell (in free and esterified forms) to the liver.
