Fig. 3.
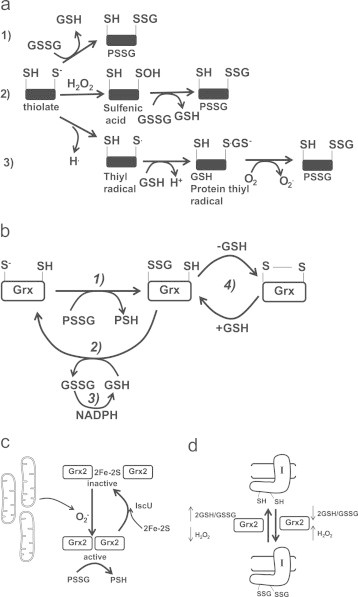
Non-enzymatic and enzymatic S-glutathionylation reactions and the modulation of Grx2. (a) Non-enzymatic glutathionylation reactions. (1) Solvent-exposed thiolate is S-glutathionylated by GSSG via a thiol disulfide exchange reaction. (2) Solvent-exposed thiolate are oxidized to a sulfenic acid residue by H2O2 which then undergoes S-glutathionylation. (3) one electron reduction of a thiol forms a thiyl radical which interacts with glutathione to form a glutathione anion thiyl radical intermediate. A disulfide bond then forms between the glutathione anion and the thiyl radical following the one electron reduction of O2 to O2−.(b) Catalytic cycle of Grx1 and Grx2 (in diagram it is simply referred to as Grx). In step 1 Grx catalyzes the rapid transfer of the gluthionyl moiety via a disulfide exchange reaction to its catalytic cysteine which produces a Grx-SSG intermediate and a deglutathionylated protein. Step 2 involves the GSH-mediated removal of the glutathionyl moiety from Grx-SSG which generates a fully reduced Grx enzyme and GSSG. For step 3 the GSSG is reduced by NADPH and glutathione reductase to regenerate GSH. Note the side reaction for Grx-SSG in step 4 an intramolecular disulfide can form in Grx. GSH is required to return Grx-SSG to its catalytic cycle. (c) Grx2 is modulated by 2Fe–2S cluster coordination and O2− mediated dissembly of the cluster. Grx2 is maintained is inactive as a homodimer. A subsequent burst of O2− results in the release of active Grx2 monomers which subsequently deglutathionylate target proteins. (d) Grx2 catalyzes the reversible S-glutathionylation of Complex I. When the 2GSH/GSSG ratio is low and H2O2 is high, Grx2 glutathionylase is activated. A high 2GSH/GSSG ratio and low H2O2 levels induce Grx2-mediated deglutathionylation of Complex I.
