Abstract
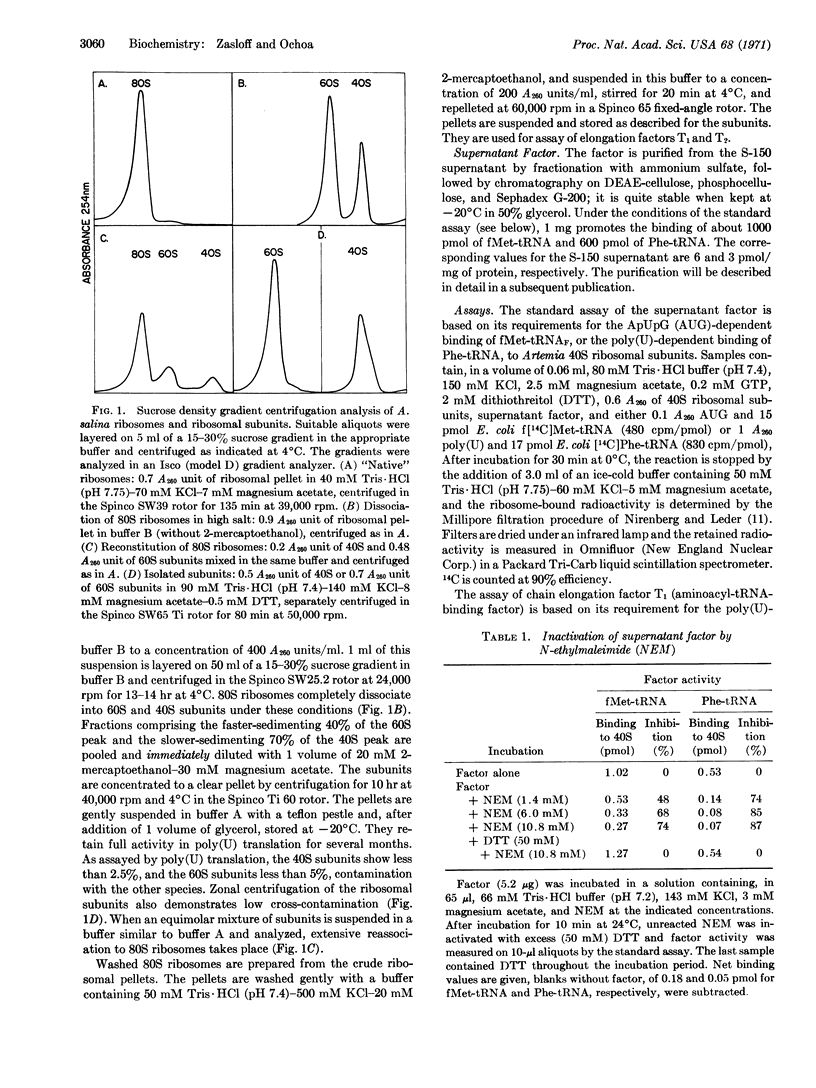
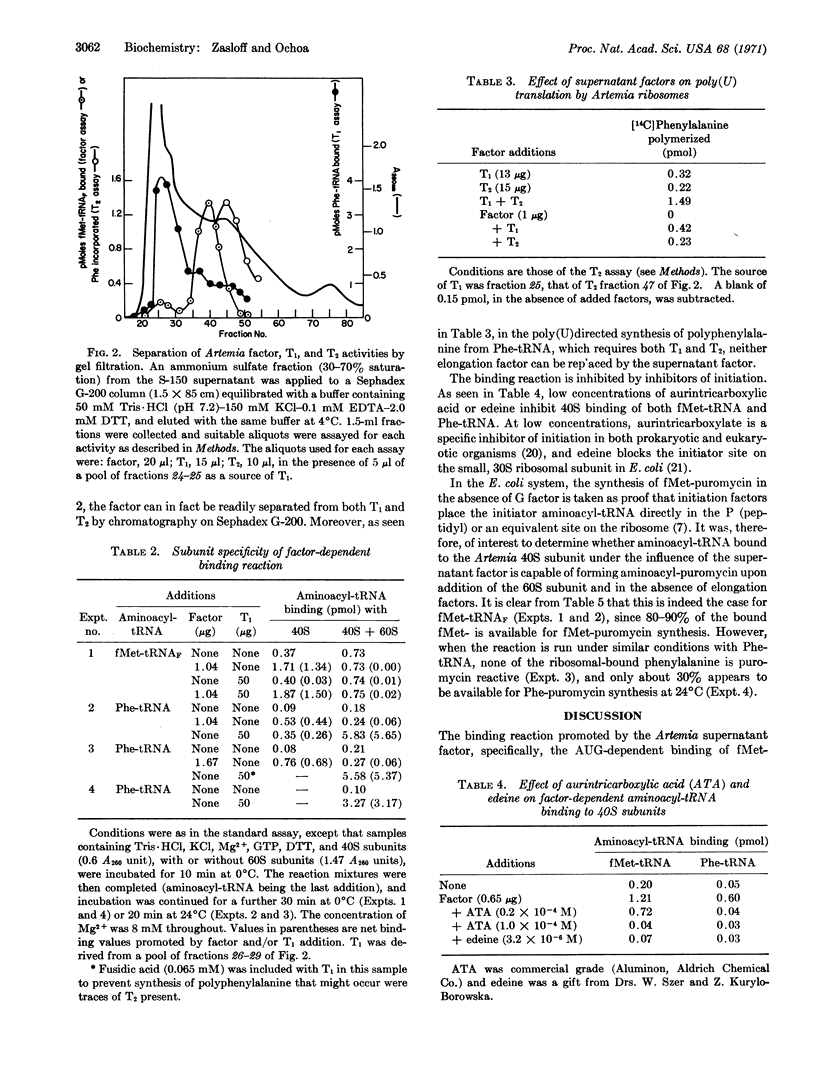
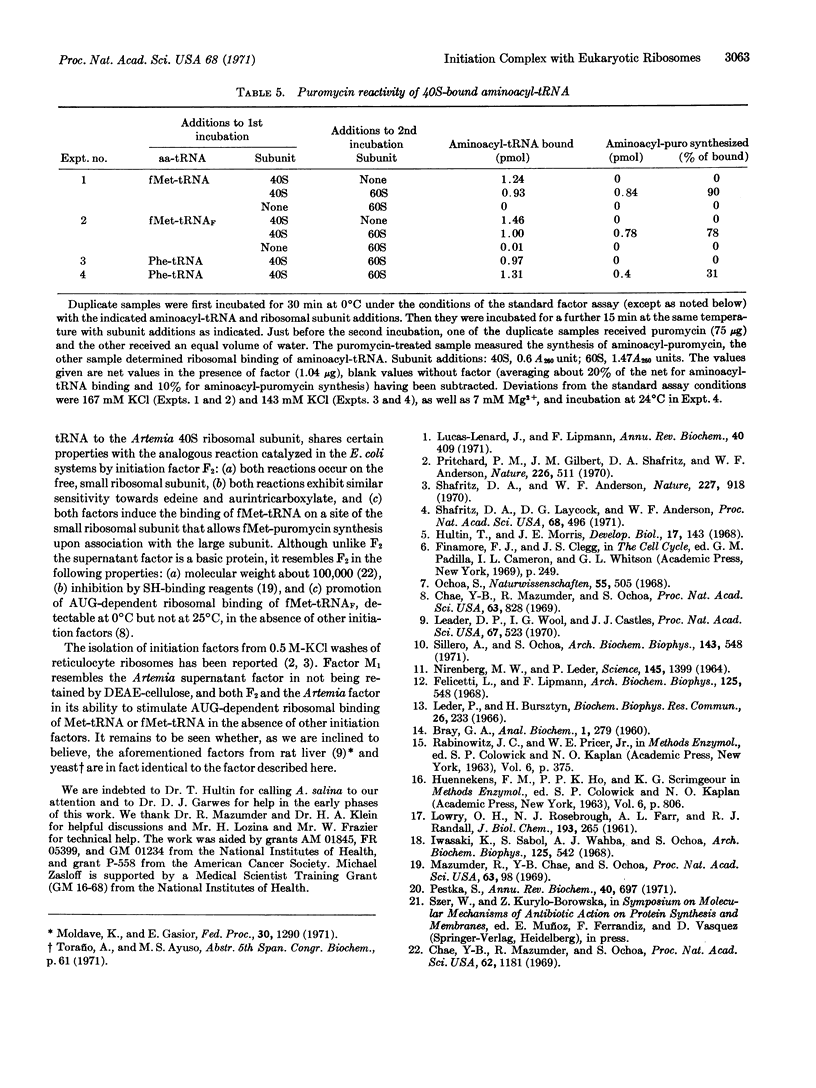
Embryos of the brine shrimp, Artemia salina, were used in a study of polypeptide chain initiation in an in vitro system from a eukaryote. A protein, isolated from the high-speed supernatant, has been highly purified and shown to have properties that suggest it is the eukaryotic equivalent of the Escherichia coli initiation factor F2: It promotes the AUG-dependent binding of fMet-tRNA (E. coli) to the Artemia 40S ribosomal subunit, but not to either the 60S or 80S species; the bound fMet-tRNA is placed in a site on the smaller subunit from which it reacts with puromycin upon addition of the 60S subunit; and the activity is sensitive to aurintricarboxylic acid and edeine, specific inhibitors of initiation. The factor, a basic protein of molecular weight about 100,000, is inactivated by N-ethylmaleimide, an SH-binding reagent, and is clearly distinct from the Artemia elongation factors, T1 and T2. In addition, the factor stimulates the poly(U)-dependent binding of Phe-tRNA (E. coli) to the Artemia 40S ribosomal subunit. This reaction, though similar to the fMet-tRNA-binding reaction, differs in that the bound Phe-tRNA is largely resistant to release by puromycin.
Keywords: Artemia salina, embryos, cysts, brine-shrimp eggs, elongation factors
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chae Y. B., Mazumder R., Ochoa S. Polypeptide chain initiation in E. coli: isolation of homogeneous initiation factor E2 and its relation to ribosomal proteins. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1181–1188. doi: 10.1073/pnas.62.4.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae Y. B., Mazumder R., Ochoa S. Polypeptide chain initiation in E. coli: studies on the function of initiation factor F1. Proc Natl Acad Sci U S A. 1969 Jul;63(3):828–833. doi: 10.1073/pnas.63.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felicetti L., Lipmann F. Comparison of amino acid polymerization factors isolated from rat liver and rabbit reticulocytes. Arch Biochem Biophys. 1968 May;125(2):548–557. doi: 10.1016/0003-9861(68)90613-9. [DOI] [PubMed] [Google Scholar]
- Hultin T., Morris J. E. The ribosomes of encysted embryos of Artemia salina during cryptobiosis and resumption of development. Dev Biol. 1968 Feb;17(2):143–164. doi: 10.1016/0012-1606(68)90058-4. [DOI] [PubMed] [Google Scholar]
- Iwasaki K., Sabol S., Wahba A. J., Ochoa S. Translation of the genetic message. VII. Role of initiation factors in formation of the chain initiation complex with Escherichia coli ribosomes. Arch Biochem Biophys. 1968 May;125(2):542–547. doi: 10.1016/0003-9861(68)90612-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leader D. P., Wool I. G., Castles J. J. A factor for the binding of aminoacyl transfer RNA to mammalian 40S ribosomal subunits. Proc Natl Acad Sci U S A. 1970 Oct;67(2):523–528. doi: 10.1073/pnas.67.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder P., Bursztyn H. Initiation of protein synthesis II. A convenient assay for the ribosome-dependent synthesis of N-formyl-C14-methionylpuromycin. Biochem Biophys Res Commun. 1966 Oct 20;25(2):233–238. doi: 10.1016/0006-291x(66)90586-9. [DOI] [PubMed] [Google Scholar]
- Lucas-Lenard J. Protein biosynthesis. Annu Rev Biochem. 1971;40:409–448. doi: 10.1146/annurev.bi.40.070171.002205. [DOI] [PubMed] [Google Scholar]
- Mazumder R., Chae Y. B., Ochoa S. Polypeptide chain initiation in E. coli: sulfhydryl groups and the function of initiation factor F2. Proc Natl Acad Sci U S A. 1969 May;63(1):98–103. doi: 10.1073/pnas.63.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIRENBERG M., LEDER P. RNA CODEWORDS AND PROTEIN SYNTHESIS. THE EFFECT OF TRINUCLEOTIDES UPON THE BINDING OF SRNA TO RIBOSOMES. Science. 1964 Sep 25;145(3639):1399–1407. doi: 10.1126/science.145.3639.1399. [DOI] [PubMed] [Google Scholar]
- Ochoa S. Translation of the genetic message. Naturwissenschaften. 1968 Nov;55(11):505–514. doi: 10.1007/BF00660121. [DOI] [PubMed] [Google Scholar]
- Prichard P. M., Gilbert J. M., Shafritz D. A., Anderson W. F. Factors for the initiation of haemoglobin synthesis by rabbit reticulocyte ribosomes. Nature. 1970 May 9;226(5245):511–514. doi: 10.1038/226511a0. [DOI] [PubMed] [Google Scholar]
- Shafritz D. A., Anderson W. F. Factor dependent binding of methionyl-tRNAs to reticulocyte ribosomes. Nature. 1970 Aug 29;227(5261):918–920. doi: 10.1038/227918a0. [DOI] [PubMed] [Google Scholar]
- Shafritz D. A., Laycock D. G., Anderson W. F. Puromycin-peptide bond formation with reticulocyte initiation factors M1 and M2. Proc Natl Acad Sci U S A. 1971 Feb;68(2):496–499. doi: 10.1073/pnas.68.2.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillero A., Ochoa S. Nuclear localization of diguanosine polyphosphates in Artemia embryos. Arch Biochem Biophys. 1971 Apr;143(2):548–552. doi: 10.1016/0003-9861(71)90239-6. [DOI] [PubMed] [Google Scholar]


