Abstract
Biological clocks are genetically encoded oscillators that allow organisms to keep track of their environment. Among them, the circadian system is a highly conserved timing structure that regulates several physiological, metabolic and behavioural functions with periods close to 24 h. Time is also crucial for everyday activities that involve conscious time estimation. Timing behaviour in the second-to-minutes range, known as interval timing, involves the interaction of cortico-striatal circuits. In this review, we summarize current findings on the neurobiological basis of the circadian system, both at the genetic and behavioural level, and also focus on its interactions with interval timing and seasonal rhythms, in order to construct a multi-level biological clock.
Keywords: biological timing, circadian system, interval timing, cortico-striatal circuits, dopamine
1. Biological timing as a multi-frequency clock
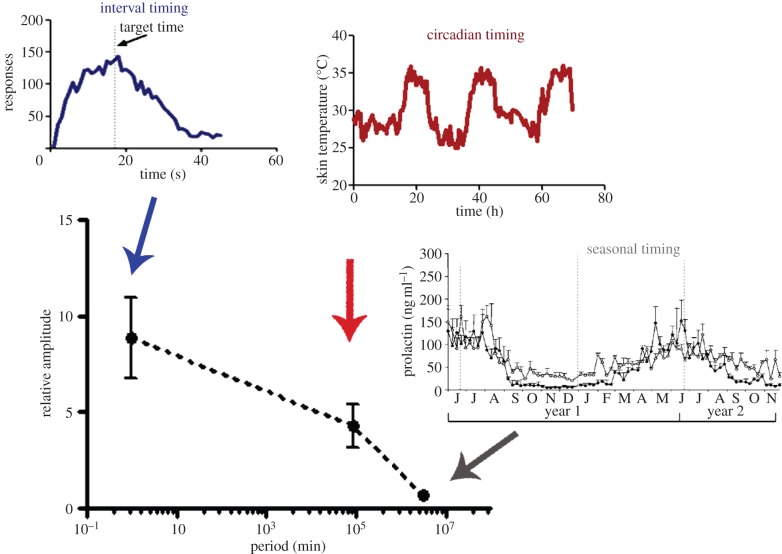
The ability to reliably sense, process and react to relevant characteristics of the physical environment is an attribute of life; indeed, when this faculty is compromised, cognitive and behavioural performance is severely impaired, because precise timing is ubiquitous and of great importance for physiology and behaviour. Biological timing includes diverse time-related processes that encompass several orders of magnitude [1,2], from microsecond processing to seasonal rhythms. Microsecond timing, for example, is crucial for sound localization, allowing animals to determine the interval it takes sound to travel from one ear to the other [3]. Millisecond timing is critical for motor control, speech generation and recognition, playing music and dancing [4] and has been proposed to depend upon a sensory-motor circuit comprising the cerebellum and the occipital, parietal and insular cortices [5]. In the seconds-to-minutes range, interval timing is involved in a number of fundamental behaviours such as foraging, decision-making and learning, via activation of cortico-striatal circuits [1]. Circadian timing refers to the 24 h range and governs a large array of physiological, metabolic and behavioural functions. In mammals, the circadian clock is located in the suprachiasmatic nucleus (SCN) of the hypothalamus [6]. Most—if not all—organisms exhibit daily and circadian rhythms with periods of ca 24 h, which also serve as the basis for seasonal-encoding mechanisms and might be related to lifespan-related processes. Finally, seasonal timing refers to rhythmic behaviour with a period which is close to the environmental annual cycle, such as reproductive or hibernation rhythms, controlled in mammals through a neuroendocrine circuit modulated by the pineal hormone melatonin [7]. Figure 1 summarizes the orders of magnitude implied in biological timing (from interval timing to seasonal rhythms), as well as the representative difference in relative amplitude, which indicates that high-frequency timing (i.e. in the seconds-to-minutes range) tends to exhibit a higher relative amplitude than low-frequency periods such as those exemplified by seasonal rhythms.
Figure 1.
Relative amplitude of biological timing. Spectrum of biological timing phenomena, from interval timing to seasonal rhythms. The main graph shows the period of such timing mechanisms, spanning about seven orders of magnitude. When relative amplitude (calculated from representative data from the available literature by dividing the average amplitude values—considered as the difference between trough and peak values—by the minimal level of the variables) is compared, a negative correlation between period and amplitude of the oscillations/behavioural responses is found. Mean ± s.d. is shown, n = 5–8. Insets—described clockwise from the top—show representative data from interval timing, circadian timing and seasonal timing, respectively. Interval timing: response rate in mice trained to respond to a 17 s interval (data from authors’ laboratory). Circadian timing: skin temperature from a 20-year-old female subject (data from authors’ laboratory). Seasonal timing: plasma prolactin levels (ng ml−1) throughout the year (adapted from [8]). (Online version in colour.)
In particular, timing oscillators in the fast (seconds–minutes) and intermediate (circadian) frequencies might share some properties, including common steps in molecular pathways that lead to the neurochemical basis of such mechanisms. There is evidence suggesting that the circadian clock may influence the rate of the interval timer; however, these relationships have not been completely elucidated at the behavioural or the molecular level. In this review, we focus on the general features of interval and, in particular, circadian timing, including genetic and molecular pathways underlying temporal coordination.
2. From very short (ultradian timing)…
Several biological mechanisms can be found in the ultradian temporal domain (less than 24 h). For example, the mammalian auditory system is a very precise sensory modality in terms of its temporal accuracy. Indeed, to spatially localize sounds coming from the left or the right, the binaural system can resolve time differences between the ears with microsecond precision. Discrimination of interaural time differences is performed by auditory midbrain neurons which are able to follow fast temporal modulations by ‘coincidence-detecting’ mechanisms [3]. Slightly longer temporal-control processes functioning in the milliseconds range, such as precise motor control, speech recognition and music perception, have been associated with cerebellar functioning [5]. In the seconds-to-minutes range, the conscious perception of time, known as interval timing, is crucial to learning, memory, decision-making and other cognitive tasks. As we shall discuss in §5, recent findings argue for the involvement of cortico-striatal circuits that are controlled by the dopaminergic modulation of oscillatory activity and lateral connectivity [9,10]. The ability to process time in the seconds-to-minutes range is impaired in patients with disorders that involve dopaminergic pathways, such as Parkinson's disease, Huntington's disease and schizophrenia [1].
A prominent temperature-compensated ultradian clock is the circa-hour (i.e. about 1 h period) that occurs from lower eukaryotes such as Saccharomyces cerevisiae [11] to mammals [12]. Recent experiments in cardiac cells reveal parallels and similarities with yeast in the dynamic and biochemical organization of ultradian redox oscillations [13]. The heartbeat mechanism has some unique features such as the activity of a dual pacemaker, e.g. a ‘membrane clock’ and a ‘calcium clock’, which might also be considered a model for molecular interactions among pacemakers [14]. Finally, fine-tuning endocrine information is coded by the circa-hour oscillation of hypothalamic–pituitary hormones, which is modulated along the oestrous or menstrual cycle, representing a complex temporal mechanism in which ultradian, circadian, infradian (i.e. several days) and even seasonal components control the neuroendocrine regulation of physiology and behaviour [12].
3. …To very long (seasonal) timing
Organisms are affected by daily and seasonal variations of many physical factors of their environment. Seasonal timing, which refers to rhythms whose period correlates with the annual environmental cycle, regulates several mechanisms, such as reproduction, diapause in insects, hibernation, fur colour changes and migration. In mammals, the pineal gland is a major component of the endocrine system that allows them to respond to the annual changes in photoperiod by adaptive alterations of their physiological state. This endocrine structure synthesizes and releases the hormone melatonin, which is secreted during the dark period of the light/dark cycle, independently of whether the animal is diurnally or nocturnally active, and the duration of the nocturnal melatonin peak is proportional to the length of the night. The brain is able to integrate photoperiodic information through these changes in duration of melatonin synthesis [7,15]. Melatonin modulates secretion of reproductive hormones by the anterior pituitary gland and also regulates the activity of the pars tuberalis [16].
It is interesting to consider that seasonal rhythmicity might be based, at least in part, on the activity of circadian pacemakers which are able to respond to photoperiod. In this sense, the SCN has also been called ‘a clock for all seasons’ regarding its ability to change circadian patterns throughout the year [17,18]. Again, intermodulation of diverse biological timing frequencies seems to be the key for temporal regulation.
4. Circadian timing: a day within the body
Circadian clocks generate self-sustaining, cell-autonomous oscillations with a time period of approximately 24 h. Features of a circadian clock in all organisms include its persistence under constant conditions, an oscillation that is temperature-compensated and its entrainment to external input. As the circadian system also modulates ultradian and infradian rhythms, in this review, we will emphasize the mechanisms of circadian clocks and rhythms.
(a). Genetics of circadian clocks
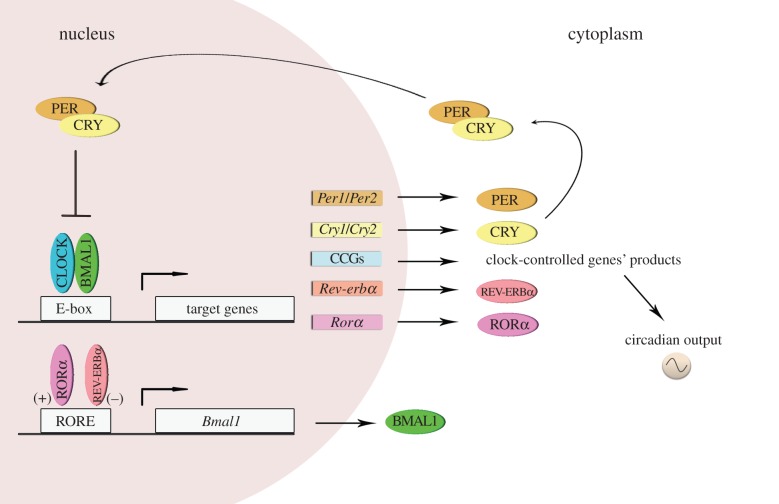
The molecular mechanism of the endogenous circadian clock comprises interlocking feedback loops involving cyclic gene products that control transcription by means of negative and positive regulation of ‘clock’ genes and proteins. The specific transcriptional/translational components differ between phylogenetic kingdoms [19]. In mammals, cell-autonomous circadian clocks are generated by a transcriptional autoregulatory feedback loop composed of the transcriptional activators CLOCK and BMAL1, and their target genes Period (Per1 and Per2) and Cryptochrome (Cry1 and Cry2). The latter (Per and Cry) are able to form a repressor complex that interacts with CLOCK/BMAL1 to inhibit their own transcription [20,21]. Post-translational events that modulate protein half-life and subcellular localization appear to contribute significantly to circadian oscillations. Several kinases and phosphatases regulate the speed, precision and function of the circadian clock [22,23]. Episodes of ubiquitination, acetylation/deacetylation and methylation also represent critical regulatory events that mediate circadian control [24–27]. Recent studies have implicated a number of microRNAs [28] and several RNA-binding protein complexes in the regulation of circadian polyadenylation, splicing, RNA stabilization and degradation [29]. Moreover, additional stabilizing feedback loops, including inhibition of Bmal1 transcription by REV-ERBα [30], further contribute to the timing and robustness of the cycle. This cycling molecular framework can also control the transcription of other genes—called clock-controlled genes (CCGs)—by acting upon specific elements in their promoter regions, for example E-boxes (illustrated in figure 2). In addition, recent information suggests that non-transcriptional oscillator components appear to be conserved across kingdoms, revealing prominent post-translational contributions to timekeeping [31–34].
Figure 2.
The molecular circadian clock in mammals. The molecular mechanisms of circadian rhythms can be illustrated by the transcription of the Period (Per1 and Per2) and Cryptochrome (Cry1 and Cry2) genes that are activated by heteromeric complexes containing CLOCK and BMAL1 proteins that act through the E-box regulatory sequences of their target genes. In turn, PER and CRY proteins inhibit BMAL1–CLOCK activity, and therefore, their own transcription. This core oscillation is augmented and stabilized by a secondary loop involving two orphan nuclear receptor proteins, REV-ERBα and RORα, which affect Bmal1 expression. The result of these complex regulatory pathways is that the mRNA and protein levels of most circadian genes oscillate with approximately 24 h period. Importantly, the CLOCK–BMAL1 heterodimer regulates the transcription of many CCGs, which in turn influence a wide array of physiological functions external to the oscillatory mechanism. (Online version in colour.)
(b). Central and peripheral clocks
In multicellular organisms, for example mammals, there is a hierarchically structured circadian system [35]. At the top of this hierarchy is the SCN, which synchronizes subordinate organ and tissue clocks using electrical, neurochemical, endocrine and metabolic signalling pathways that impact the molecular mechanisms of cellular oscillators. The SCN consists of paired nuclei located in the ventral hypothalamus. In rats, each nucleus contains approximately 10 000 neurons, characterized by a small size and a high density [36]. Circadian SCN rhythmicity can be recorded at tisular level and is an emergent property of individual oscillators working together in synchrony [37]. Individual SCN cells have circadian rhythms in electrical activity, but with diverse period and phase [38,39]. Recent data highlight that, when isolated, SCN neurons exhibit a range of behaviours including damped or unstable circadian oscillations [40,41]. In this sense, several computational models have been proposed to understand the complex behaviours of SCN cells [42].
Shortly after the discovery of clock genes in mammals, it became evident that circadian clocks are present not only in SCN neurons but also in most, if not all, peripheral tissues. These peripheral clocks appear to have a molecular clockwork similar to the one described for the SCN. Thus, in both SCN cells and peripheral cells, the rhythm-generating molecular circuitry is based upon transcriptional/translational feedback loops involving essentially the same core clock components [43], although some variations are present, for example the neuronal PAS domain protein 2 (NPAS2) acting as a functional substitute for CLOCK in some brain areas [44,45]. Moreover, there are at least two circadian oscillators—a food-entrainable oscillator (FEO) and a methamphetamine (MAP)-entrainable oscillator (MASCO)—which are independent from the SCN and will be discussed in §4c.
(c). Synchronization of circadian clocks
The circadian clock is a self-sustained biological oscillator with a period close to 24 h in constant conditions. Circadian clocks in nature are normally exposed to a rhythmic environment, so that appropriate signals (Zeitgebers, from German Zeit, ‘time’; geben, ‘to give’), such as light, temperature or food, synchronize its oscillation [46]. In mammals, the most powerful synchronizer is the daily light/dark cycle. Light stimulates a group of photosensitive retinal ganglion cells that express the photopigment melanopsin [47] and project to the SCN through the retinohypothalamic tract. Glutamate and pituitary adenylate cyclase-activating polypeptide (PACAP) are the primary neurotransmitters responsible for mediating the synchronizing properties of light, and act upon NMDA and AMPA/kainate receptors for glutamate and on the PACAP-specific receptor (PAC1). This leads to an increase of the intracellular concentrations of Ca2+, which initiates a signal transduction cascade in SCN neurons that ultimately results in a phase shift of the circadian system [46,48–50].
The circadian pacemaker varies in its temporal responsiveness to external stimuli. For example, in nocturnal rodents, exposure to light synchronizes circadian rhythms by inducing phase delays during the early night and phase advances during the late night, led by diverse signal transduction pathways which ultimately rely on the activation of transcription factors such as CREB and clock genes [51]. During the late night, when light induces phase advances of behavioural rhythms, photic stimulation specifically activates the guanylyl cyclase/cGMP/cGMP-dependent kinase (PKG) pathway [49,52]. Therefore, the accessibility of specific signalling pathways is fundamental for regulation of circadian timing.
Other entrainment cues, such as food intake, temperature, drugs or even social interaction, can reset circadian rhythms, eliciting phase shifts mainly during the subjective day (the resting period in rodents). Non-photic synchronization influences the SCN via two major input pathways, the geniculohypothalamic tract (GHT), and serotonergic (5HT) input from the dorsal raphe nucleus (DRN) and median raphe nucleus (MRN) [35]. However, two non-photically entrained circadian oscillators have been reported—a FEO and a MASCO—which are independent of the SCN [53,54]. When food availability is limited to a few hours during each day, mammals quickly alter daily rhythms of physiology and behaviour, such as locomotor activity, body temperature and corticosterone secretion, to correlate with the food availability rhythm [55]. Under these circumstances, a clear behavioural output is food-anticipatory activity (FAA), which implies an increase in locomotor activity that occurs before a daily timed meal. This food-entrainable oscillator is independent of the SCN and still displays clear circadian characteristics. One of its most important features is that FAA persists in the absence of food, suggesting that the FEO is able to generate a sustained free-running rhythm [53]. Similarly, the effects of MAP on circadian rhythms suggest the presence of an SCN-independent, MAP-sensitive circadian oscillator [54]. Nevertheless, the anatomical loci of these two oscillators are unknown (despite exhaustive searches for the FEO; [56]). It is worth noting that at least some features of circadian entrainment (such as non-photic synchronization induced by forced locomotion, feeding or neurochemical stimulation by metamphetamine and other agents) also depend upon reward-related mechanisms, including dopaminergic activation.
(d). Circadian clocks in metabolism, mood-related disorders and cognition
Food intake operates as a powerful Zeitgeber for entrainment of peripheral oscillators. Therefore, there is a tightly coupled relationship between metabolic state and the circadian clock, both at molecular and physiological levels [57]. Cycling of circadian components controls fundamental cellular and metabolic processes, including gluconeogenesis, oxidative phosphorylation, RNA processing and translation. Moreover, the SCN regulates circadian rhythms in leptin, plasma glucose, glucose tolerance, corticosteroids and cardiovascular function via neural and/or humoral signals to the white adipose tissue, liver, pancreas, adrenal cortex and heart [58]. Various hormones and metabolites that are generated by peripheral clocks feed back to the SCN, influencing specific brain functions [57,59]. Indeed, several of the core clock components function as ‘redox sensors’ which bind to nicotinamide adenine dinucleotide (NAD+) and are modulated by the histone deacetylase SIRT1, both of which are influenced greatly by the nutrient levels in the organism [59].
Several lines of research suggest that alignment between central behavioural rhythms and feeding time is important in metabolic health. Animals fed a high-fat diet shift their pattern of food intake and consume nearly all of the excess calories at the incorrect circadian time, that is, during the rest period [60]. A similar misalignment of feeding between activity and rest is observed in Clock-mutant [61] and Npas2-knockout [43] mice, and a high-fat diet induces period lengthening in wild-type mice [60]. Similarly, mice fed with a high-fat diet exclusively during the rest period have accelerated weight gain as compared with animals fed during the correct circadian time [62]. Recent work indicates that the circadian clock in adipocytes affects the feeding rhythm of mice, which in turn exerts a profound influence on whole-body energy homoeostasis. Thus, adipocyte-specific deletion of a core circadian clock gene, Arntl (Bmal1), in mice shifts the timing of their feeding behaviour, resulting in obesity [63]. In humans, chronic circadian misalignment has been proposed to be the underlying cause for the adverse metabolic and cardiovascular health effects of shift work [64]. Information related to genetic associations between circadian timing and metabolic disorders is shown in table 1.
Table 1.
Genetic associations between circadian timing and cognitive, metabolic or mood-related disorders.
| gene | clinical association/alteration | reference | ||
|---|---|---|---|---|
| mood-related disorders | humans | ARNTL | bipolar disorder and seasonal affective/winter depression | [65–67] |
| CLOCK | bipolar disorder and major depressive disorder | [68–71] | ||
| CRY1 | bipolar disorder and major depressive disorder | [69] | ||
| CRY2 | bipolar disorder and seasonal affective/winter depression | [72,73] | ||
| NPAS2 | bipolar disorder, major depressive disorder and seasonal affective/winter depression | [69,74] | ||
| NR1D1 | bipolar disorder | [75] | ||
| PER2 | depression and seasonal affective/winter depression | [67,76] | ||
| PER3 | bipolar disorder and schizoaffective disorder | [65,66,77] | ||
| RORA | major depressive disorder | [76] | ||
| RORB | bipolar disorder (pediatric) | [78] | ||
| animals | CLOCK, CLK, DBT and PER mutants in Drosophila | lack of cocaine sensitization | [79] | |
| CLOCK-d19 mice | mania-like behaviour | [80] | ||
| PER2-deficient mice | reduced depression- and anxiety-like behaviours | [45] | ||
| RORB-deficient mice | reduced depression- and anxiety-like behaviours | [81] | ||
| metabolic disorders | humans | RAI1 | obesity in Smith–Magenis syndrome | [82] |
| CRY2 and melatonin receptor (MTNR) 1B | type-2 diabetes risk | [83] | ||
| animals | CLOCK-d19 mice | obesity and metabolic syndrome similar to diabetes | [61] | |
| NPAS2-deficient mice | adaptability to food restriction | [44] | ||
| cognitive disorders | humans | RAI1 | intellectual deficit | [82] |
| animals | PER mutants in Drosophila | defective in long-term memory formation | [84] | |
| CRY1-CRY2 mutant mice | disrupted time–place learning | [85] |
Animals with mutations or disruptions in the circadian machinery show changes in mood and behaviour. In mice, the Δ19 mutation in the Clock gene is accompanied by a spectrum of behavioural abnormalities including mania and hyperactivity [80]. Additionally, these animals as well as animals carrying mutations in the Per genes (mPer1Brdm1 and mPer2Brdm1 mutant mice) display altered sensitization to, and preference for, drugs of abuse such as cocaine [86,87] and alcohol [88,89]. Clock gene mutations appear to affect the dopaminergic system [45,80], among other neurochemical systems [89]. Also, mice deficient in the D2 receptor show aberrant light masking (light-induced suppression of locomotion) [90], and dopamine (DA) can regulate circadian expression of Clock genes [91,92]. These data suggest that defects in the clock system can alter DA synthesis and metabolism and might thereby contribute to depression-like symptoms. In humans, alterations in metabolic homoeostasis have been associated with mood disorders [93]. For example, together with metabolic syndrome, chronic shift work may favour the development of mood disorders [94], probably due to a misalignment of rhythms in body temperature, melatonin and sleep [95]. Conversely, individuals that suffer from mood disorders benefit from predictable daily routines including strictly followed bed- and mealtime [96]. These routines probably help to entrain and synchronize peripheral clocks in the body to maintain the integrity of the circadian system and physiology [97]. A typical mood disorder related to misalignment between environmental external and body internal rhythms is seasonal affective disorder (SAD), which is characterized by depressive symptoms that occur during the winter [98]. Phase delays in circadian secretion of cortisol and melatonin at specific times of the day have been observed in patients with either SAD or major depression, which further links the clock on a molecular level with these diseases [99]. Light therapy is an efficient method for the treatment of SAD [100], suggesting a connection between light-sensitive pathological mood states and light-sensitive circadian clocks. Disruptions in circadian rhythms have also been associated with bipolar disorder (BD) and major depressive disorder (MDD). Both BD and MDD involve deficits in reward processing and motivation, which are modulated by the circadian clock [101]. A number of polymorphisms in human circadian genes have been correlated with the incidence of BD and MDD (table 1). Moreover, antidepressants and mood stabilizers have been reported to entrain the circadian clock, which may affect drug efficacy, and therefore treatment viability [51]. Future studies will bring more light into the specific role of circadian clocks in the contribution to the pathophysiology of mood disorders, with the possibility of improving clinical treatments.
There is evidence that cognitive performance and learning may be influenced by circadian processes [102–104], and recent information reveals that regularly timed cognitive processes impact circadian rhythms [105], indicating a bidirectional interaction between cognitive performance and circadian processes. Furthermore, at least for some tasks in rodents, night-phase performance can never be equalled by light-phase performance regardless of the strength of entrainment to the schedule, which may have deep implications for shift work therapies [101]. In addition, desynchrony between internal and environmental time has been associated with impaired cognitive function in animals [106–108] and humans [109–112]. Although it is well established that cognitive abilities vary as a function of time of day, there is still a widespread view that the circadian clock has a singular role in cognition related to the control of the timing of sleep, and that the main factor for cognitive maintenance is the quality and duration of sleep. However, there is also evidence that indicates a circadian control of cognition beyond sleep timing, as revealed by forced desynchrony protocols [113,114].
5. Circadian clocks and interval timing: as time goes by
A fundamental component of cognition is the perception of the passage of time. As already stated, interval timing involves short-time estimation in the seconds-to-minutes range [1]. Striatal medium spiny neurons detect the coincident activity of specific beat patterns of cortical oscillations [9,10]. DA signalling has been shown to be involved in the regulation of interval timing speed, because DA receptor agonists or antagonists are able to shift the perception of the signal duration. In particular, striatal DA type 2 (D2) receptor plays an important role in the modulation of interval timing [115]. Activation of NMDA-type glutamate receptors is also critical for interval timing mechanisms [116,117]. In addition, recent studies of molecular genetics have demonstrated the importance of specific DA regulators on interval timing performance, for example the DRD2/ANKK1-Taq1a, which is a D2 receptor polymorphism associated with decreased D2 density in the striatum, and the genes regulating the catechol-O-methyltransferase (COMT) enzyme, which degrades catecholamines in the frontal cortex (table 2).
Table 2.
Genetic associations related to interval timing disorders.
| gene | clinical association/alteration | reference | |
|---|---|---|---|
| humans | catechol-O-methyltransferase (COMT Val158Met polymorphism) | interval timing impaired in the suprasecond range and effect of reward magnitude | [118,119] |
| dopamie receptor type 2 (DRD2/ANKK1-Taq1a polymorphism) | poorer performance with perceptual timing task and effect of reward magnitude | [118,119] | |
| animals | DAT−/− mice | complete loss of temporal control. Hyperactivity and learning impairment; insensitive to psychostimulants | [120] |
| D2R transgenic mice | impairment in timing accuracy and precision. Impairment in tasks that require working memory and behavioural flexibility | [115] |
Current evidence suggests that the internal clock which mediates the perception of short durations is sensitive to temperature, attention, emotions, drug and diet manipulations [121,122], all of which can be modulated by the circadian system. Indeed, time-of-day effects have been observed for the timing of auditory and visual signals in the seconds-to-minutes range [123–126]. For example, several studies have shown that time judgements in humans covary with normal circadian rhythms [127,128]. Consistent with this finding, a circadian rhythm in time estimates was documented in control subjects, but it was found to be disrupted in shift workers [125]. Moreover, rats exhibit circadian variations in time perception similar to those that have been demonstrated in humans [129]. Furthermore, it was reported that sleep deprivation influences diurnal variation of time estimation in humans [130]. In Drosophila melanogaster, timing of short intervals is disrupted in circadian mutants for each of the three allelic per mutations, pers, perl and pero [131]. Recently, significant differences in the estimation of 24 s intervals at different times of day were reported in mice [132]. These differences were maintained under constant dark (DD) conditions, but impaired in mice under constant light (LL) conditions which abolish circadian rhythmicity. Moreover, short-time estimation in animals subjected to a 6 h advance of the light/dark cycle was transiently affected, indicating that temporal desynchronization of the circadian system is able to negatively affect time estimation. Taken together, these results suggest that short-time estimation is modulated by the circadian clock.
Figure 3 summarizes the principal molecular mechanisms supporting the various ways in which the circadian system interacts with interval timing. The main dopaminergic input to the striatum comes from the substantia nigra (SN) and the ventral tegmental area (VTA), and previous studies [133,134] have demonstrated the expression of circadian clock genes in these structures. The protein products of these clock genes act as transcription factors through binding to specific elements in promoter regions, such as E-boxes and RORE elements. These sequences have been found in the promoter region of components involved in dopaminergic metabolism, such as DA transporter (DAT), tyrosine hydroxylase (TH) and monoamine oxidase (MAO), suggesting that the expression of these components is under circadian regulation [45,135,136]. Some of them, like MAO, also exhibit diurnal rhythms in enzymatic activity [45]. Moreover, 24 h rhythms in DA levels and dopaminergic transmission were reported in both the striatum and nucleus accumbens [91,137]. In particular, the expression of DAT and TH showed a daily rhythm which was abolished in SCN-lesioned rats [138]. There is also evidence that indicate the regulation of DA receptors by circadian clock proteins in the striatum. In this sense, a 24 h rhythm in the expression of the DA D3 receptor was recently described, which is enhanced by RORα and inhibited by Rev-Erbα [139]. Moreover, a recent study [140] in humans suggested that a polymorphism in the PER2 protein correlates with striatal D2 receptor availability. Reciprocally, the blockade of D2 receptors blunted the rhythm of striatal PER2, and daily activation of D2 receptor restored and entrained the PER2 rhythm in a DA-depleted striatum in rats [87]. Moreover, quinpirole, a D2 receptor agonist, inhibited CLOCK and PER1 expression in the mouse striatum [141]. Taken together, these data suggest a circadian regulation of dopaminergic transmission in striatal circuits. This interaction at dopaminergic level could be in part responsible for the crosstalk between the circadian system and short-time estimation.
Figure 3.
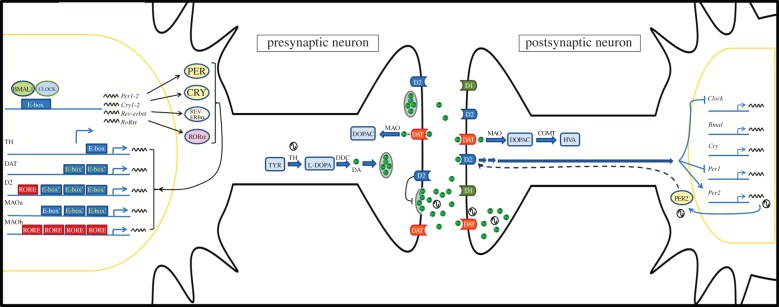
Molecular mechanisms for circadian modulation of interval timing. The circadian system control dopaminergic transmission at both presynaptic and postsynaptic levels. In presynaptic neurons—such as VTA or SN dopaminergic neurons—circadian clock proteins generate daily rhythms in the expression of components related to dopaminergic neurotransmission, mainly by acting as transcription factors through binding to E-boxes and ROR elements from promoter regions. Thus, the circadian control of dopaminergic enzymes could be involved either in rhythmic DA synthesis by TH, rhythmic DA release—under control of D2 autoreceptors—or rhythmic degradation mediated by DAT and MAO. On the other hand, in postsynaptic neurons—such as striatal medium spiny neurons—multiple levels of control by circadian components exist. There are daily rhythms in DAT expression, DA content and D2 receptor availability. In turn, dopaminergic function regulates the expression of clock genes through the activation of D2 receptors. COMT, Catechol-O-methyl transferase; D1, DA receptor type 1; D2, DA receptor type 2; DA, dopamine; DAT, DA transporter; DDC, DOPA decarboxylase; DOPAC, 3,4-dihydroxyphenylacetic acid; E-box′, non-canonical e-box; HVA, homovanillic acid; MAO, monoamine oxidase; ROR, retinoid-related orphan receptor; RORE, ROR response element; SN, substantia nigra; TH, tyrosine hydroxylase; TYR, tyrosine; VTA, ventral tegmental area. (Online version in colour.)
6. Constructing a multi-level biological clock
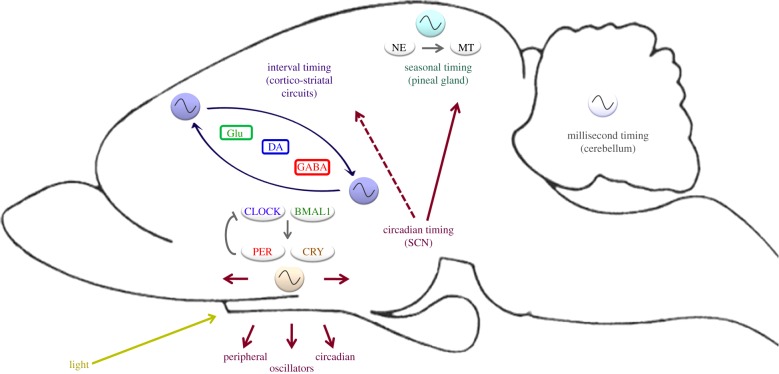
Several oscillators in the brain are involved in regulating timing behaviour in different temporal scales (figure 4). The interaction between circadian timing and other scales of biological timing—such as interval/circadian timing, or circadian/seasonal timing—may reflect an adaptive mechanism for living in a planet that rotates every 24 h. This ‘multi-level’ biological clock may contribute as a whole to human and animal behaviour.
Figure 4.
A network of oscillators regulates timing behaviour. The model represents mechanisms responsible for seasonal, circadian, interval and millisecond timing. In seasonal timing, the pineal gland allows mammals to respond to the annual changes in photoperiod by adaptive alterations of their physiological state. Melatonin, the major hormone produced by the pineal gland, displays daily and seasonal patterns of secretion which synchronize numerous physiological processes in photoperiodic species. In the range of approximately 24 h, circadian timing operates through a self-sustaining oscillator whose location in mammals is the SCN in the hypothalamus. The SCN controls several endocrine, metabolic and behavioural functions through peripheral oscillators. In the seconds-to-minutes range, interval timing depends upon cortico-striatal circuits. In the higher frequency range, millisecond timing has been proposed to depend upon a sensory-motor network that includes the cerebellum. These different oscillators can interact between themselves. Thus, release of melatonin by the pineal gland is mainly driven by the circadian clock, which controls the release of norepinephrine from the dense pineal sympathetic afferents. The circadian system could also modulate interval timing, perhaps through regulation of DA rhythms in the brain. BMAL1, brain and muscle ARNT-like 1; CLOCK, circadian locomotor output cycles kaput; CRY, cryptochrome; DA, dopamine; GABA, γ-aminobutyric acid; Glu, glutamate; MT, melatonin; NE, norepinephrine; PER, period. (Online version in colour.)
We propose that at least some of the oscillators related to biological timing are linked by neurochemical/molecular common steps. Besides the well-known interaction between circadian and seasonal rhythmicity [17,142,143] in which the SCN was proposed as a ‘clock for all seasons’ by controlling annual or seasonal activity of the neuroendocrine axis, notably by modulating pineal melatonin secretion both daily and throughout the year, here we suggest expanding this analogy to a ‘multi-level biological clock’ as a systemic approach to biological timing.
We have described in some detail the neurochemical and molecular bidirectional links between circadian and interval timers. Indeed, there is evidence for both time-of-day modulation of subjective time estimation processes and, conversely, for the regulation of the molecular circadian clock by neurochemical agents which are key actors of seconds-to-minutes timing. A common feature between these two systems is encoded by the reward-related mechanisms in the brain, which might function as powerful Zeitgebers of the circadian clock, and also provide a motivational framework underlying some of the main characteristics of interval timing. Pharmacological and molecular evidence suggests that dopaminergic homoeostasis might be regarded as a missing link between seconds, minutes and eventually days. A multi-level biological clock (for all seasons?) might be at the core of our timing capabilities and could fine-tune our appreciation of time.
In summary, temporal organization is a hallmark of life as an adaptation to environmental cycles and the ability to predict changes in the near future. Temporal misalignment may have severe consequences on physiology and behaviour which should be taken into account when designing work, school and health schedules. Current and future research on biological timing will certainly lead to an improvement in social temporal organization, leading to a better quality of life across the lifespan. Individuals, and societies, are the stuff of which time is made.
Funding statement
Studies in the authors’ laboratory were supported by the National Science Agency (ANPCyT), the National Research Council (CONICET) and the National University of Quilmes.
References
- 1.Buhusi CV, Meck WH. 2005. What makes us tick? Functional and neural mechanisms of interval timing. Nat. Rev. Neurosci. 6, 755–765. ( 10.1038/nrn1764) [DOI] [PubMed] [Google Scholar]
- 2.Buonomano DV. 2007. The biology of time across different scales. Nat. Chem. Biol. 3, 594–597. ( 10.1038/nchembio1007-594) [DOI] [PubMed] [Google Scholar]
- 3.Siveke I, Ewert SD, Grothe B, Wiegrebe L. 2008. Psychophysical and physiological evidence for fast binaural processing. J. Neurosci. 28, 2043–2052. ( 10.1523/JNEUROSCI.4488-07.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mauk MD, Buonomano DV. 2004. The neural basis of temporal processing. Annu. Rev. Neurosci. 27, 307–340. ( 10.1146/annurev.neuro.27.070203.144247) [DOI] [PubMed] [Google Scholar]
- 5.Bueti D, Lasaponara S, Cercignani M, Macaluso E. 2012. Learning about time: plastic changes and interindividual brain differences. Neuron 75, 725–737. ( 10.1016/j.neuron.2012.07.019) [DOI] [PubMed] [Google Scholar]
- 6.Dunlap JC, Loros JJ, DeCoursey PJ. 2004. Chronobiology. Biological timekeeping. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 7.Prendergast BJ, Nelson RJ, Zucker I. 2002. Mammalian seasonal rhythms: behavior and neuroendocrine substrates. Horm. Brain Behav. 2, 93–156. [Google Scholar]
- 8.Gómez-Brunet A, Santiago-Moreno J, del Campo A, Malpaux B, Chemineau P, Tortonese DJ, Gonzalez-Bulnes A, López-Sebastián A. 2008. Endogenous circannual cycles of ovarian activity and changes in prolactin and melatonin secretion in wild and domestic female sheep maintained under a long-day photoperiod. Biol. Reprod. 78, 552–562. ( 10.1095/biolreprod.107.064394) [DOI] [PubMed] [Google Scholar]
- 9.Matell MS, Meck WH. 2004. Cortico-striatal circuits and interval timing: coincidence-detection of oscillatory processes. Cogn. Brain. Res. 21, 139–170. ( 10.1016/j.cogbrainres.2004.06.012) [DOI] [PubMed] [Google Scholar]
- 10.Meck WH. 2006. Neuroanatomical localization of an internal clock: a functional link between mesolimbic, nigrostriatal, and mesocortical dopaminergic systems. Brain Res. 1109, 93–107. ( 10.1016/j.brainres.2006.06.031) [DOI] [PubMed] [Google Scholar]
- 11.Lloyd D, Murray DB, Klevecz RR, Wolf J, Kuriyama H. 2008. The ultradian clock (∼40 min) in yeast (Saccharomyces cerevisiae). In Ultradian rhythms from molecules to mind (eds Lloyd D, Rossi EL.), pp. 11–42. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 12.Stupfel M. 1992. Metabolic and behavioural long period ultradian rhythms in endotherms. In Ultradian rhythms in life processes: an inquiry into fundamental principles of chronobiology and psychobiology (eds Lloyd D, Rossi EL.), pp. 207–239. New York, NY: Springer. [Google Scholar]
- 13.Lloyd D, Cortassa S, O'Rourke B, Aon MA. 2012. What yeast and cardiomyocytes share: ultradian oscillatory redox mechanisms of cellular coherence and survival. Integr. Biol. 4, 65–74. ( 10.1039/c1ib00124h) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monfredi O, Maltsev VA, Lakatta EG. 2013. Modern concepts concerning the origin of the heartbeat. Physiology 28, 74–92. ( 10.1152/physiol.00054.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pévet P. 2003. Melatonin in animal models. Dialogues Clin. Neurosci. 5, 343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dardente H, Wyse CA, Birnie MJ, Dupré SM, Loudon AS, Lincoln GA, Hazlerigg DG. 2010. A molecular switch for photoperiod responsiveness in mammals. Curr. Biol. 20, 2193–2198. ( 10.1016/j.cub.2010.10.048) [DOI] [PubMed] [Google Scholar]
- 17.VanderLeest HT, Houben T, Michel S, Deboer T, Albus H, Vansteensel MJ, Block GD, Meijer JH. 2007. Seasonal encoding by the circadian pacemaker of the SCN. Curr. Biol. 17, 468–473. ( 10.1016/j.cub.2007.01.048) [DOI] [PubMed] [Google Scholar]
- 18.Pittendrigh CS, Daan S. 1976. A functional analysis of circadian pacemakers in nocturnal rodents. I. The stability and lability of spontaneous frequency. J. Comp. Physiol. 106, 223–252. ( 10.1007/BF01417856) [DOI] [Google Scholar]
- 19.Lakin-Thomas PL. 2006. Transcriptional feedback oscillators: maybe, maybe not. J. Biol. Rhythms 21, 83–92. ( 10.1177/0748730405286102) [DOI] [PubMed] [Google Scholar]
- 20.Reppert SM, Weaver DR. 2002. Coordination of circadian timing in mammals. Nature 418, 935–941. ( 10.1038/nature00965) [DOI] [PubMed] [Google Scholar]
- 21.Takahashi JS, Hong HK, Ko CH, McDearmon EL. 2008. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat. Rev. Genet. 9, 764–775. ( 10.1038/nrg2430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallego M, Virshup DM. 2007. Post-translational modifications regulate the ticking of the circadian clock. Nat. Rev. Mol. Cell Biol. 8, 139–148. ( 10.1038/nrm2106) [DOI] [PubMed] [Google Scholar]
- 23.Vanselow JT, Kramer A. 2010. Posttranslational regulation of circadian clocks. In The circadian clock (ed. Albrecht U.), pp. 79–104. New York, NY: Springer. [Google Scholar]
- 24.Hirayama J, Sahar S, Grimaldi B, Tamaru T, Takamatsu K, Nakahata Y, Sassone-Corsi P. 2007. CLOCK-mediated acetylation of BMAL1 controls circadian function. Nature 450, 1086–1090. ( 10.1038/nature06394) [DOI] [PubMed] [Google Scholar]
- 25.Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. 2008. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 134, 329–340. ( 10.1016/j.cell.2008.07.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ripperger JA, Merrow M. 2011. Perfect timing: epigenetic regulation of the circadian clock. FEBS Lett. 585, 1406–1411. ( 10.1016/j.febslet.2011.04.047) [DOI] [PubMed] [Google Scholar]
- 27.Valekunja UK, Edgar RS, Oklejewicz M, van der Horst GT, O'Neill JS, Tamanini F, Turner DJ, Reddy AB. 2013. Histone methyltransferase MLL3 contributes to genome-scale circadian transcription. Proc. Natl Acad. Sci. USA 110, 1554–1559. ( 10.1073/pnas.1214168110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng HY, et al. 2007. microRNA modulation of circadian-clock period and entrainment. Neuron 54, 813–829. ( 10.1016/j.neuron.2007.05.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pegoraro M, Tauber E. 2008. The role of microRNAs (miRNA) in circadian rhythmicity. J. Genet. 87, 505–511. ( 10.1007/s12041-008-0073-8) [DOI] [PubMed] [Google Scholar]
- 30.Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U. 2002. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110, 251–260. ( 10.1016/S0092-8674(02)00825-5) [DOI] [PubMed] [Google Scholar]
- 31.Nakajima M, Imai K, Ito H, Nishiwaki T, Murayama Y, Iwasaki H, Oyama T, Kondo T. 2005. Reconstitution of circadian oscillation of cyanobacterial KaiC phosphorylation in vitro. Science 308, 414–415. ( 10.1126/science.1108451) [DOI] [PubMed] [Google Scholar]
- 32.O'Neill JS, van Ooijen G, Dixon LE, Troein C, Corellou F, Bouget FY, Reddy AB, Millar AJ. 2011. Circadian rhythms persist without transcription in a eukaryote. Nature 469, 554–558. ( 10.1038/nature09654) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reddy AB, et al. 2006. Circadian orchestration of the hepatic proteome. Curr. Biol. 16, 1107–1115. ( 10.1016/j.cub.2006.04.026) [DOI] [PubMed] [Google Scholar]
- 34.O'Neill JS, Reddy AB. 2011. Circadian clocks in human red blood cells. Nature 469, 498–503. ( 10.1038/nature09702) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Albrecht U. 2012. Timing to perfection: the biology of central and peripheral circadian clocks. Neuron 74, 246–260. ( 10.1016/j.neuron.2012.04.006) [DOI] [PubMed] [Google Scholar]
- 36.Antle MC, Silver R. 2005. Orchestrating time: arrangements of the brain circadian clock. Trends Neurosci. 28, 145–151. ( 10.1016/j.tins.2005.01.003) [DOI] [PubMed] [Google Scholar]
- 37.Karatsoreos IN, Silver R. 2007. Minireview: the neuroendocrinology of the suprachiasmatic nucleus as a conductor of body time in mammals. Endocrinology 148, 5640–5647. ( 10.1210/en.2007-1083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welsh DK, Logothetis DE, Meister M, Reppert SM. 1995. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron 14, 697–706. ( 10.1016/0896-6273(95)90214-7) [DOI] [PubMed] [Google Scholar]
- 39.Herzog ED, Schwartz WJ. 2002. A neural clockwork for encoding circadian time. J. Appl. Physiol. 92, 401–408. ( 10.1152/japplphysiol.00836.2001) [DOI] [PubMed] [Google Scholar]
- 40.Webb AB, Angelo N, Huettner JE, Herzog ED. 2009. Intrinsic, nondeterministic circadian rhythm generation in identified mammalian neurons. Proc. Natl Acad. Sci. USA 106, 16 493–16 498. ( 10.1073/pnas.0902768106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ko CH, et al. 2010. Emergence of noise-induced oscillations in the central circadian pacemaker. PLoS Biol. 8, e1000513 ( 10.1371/journal.pbio.1000513) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Webb AB, Taylor SR, Thoroughman KA, Doyle FJ, 3rd, Herzog ED. 2012. Weakly circadian cells improve resynchrony. PLoS Comput. Biol. 8, e1002787 ( 10.1371/journal.pcbi.1002787) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dibner C, Schibler U, Albrecht U. 2010. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 72, 517–549. ( 10.1146/annurev-physiol-021909-135821) [DOI] [PubMed] [Google Scholar]
- 44.Dudley CA, Erbel-Sieler C, Estill SJ, Reick M, Franken P, Pitts S, McKnight SL. 2003. Altered patterns of sleep and behavioral adaptability in NPAS2-deficient mice. Science 301, 379–383. ( 10.1126/science.1082795) [DOI] [PubMed] [Google Scholar]
- 45.Hampp G, Albrecht U. 2008. The circadian clock and mood-related behavior . Commun. Integr. Biol. 1, 1–3. ( 10.4161/cib.1.1.6286) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Golombek DA, Rosenstein RE. 2010. Physiology of circadian entrainment. Physiol. Rev. 90, 1063–1102. ( 10.1152/physrev.00009.2009) [DOI] [PubMed] [Google Scholar]
- 47.Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, Hogenesch JB, Provencio I, Kay SA. 2002. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science 298, 2213–2216. ( 10.1126/science.1076848) [DOI] [PubMed] [Google Scholar]
- 48.Golombek DA, Ferreyra GA, Agostino PV, Murad AD, Rubio MF, Pizzio GA, Katz ME, Marpegan L, Bekinschtein TA. 2003. From light to genes: moving the hands of the circadian clock. Front. Biosci. 8, s285–s293. ( 10.2741/1038) [DOI] [PubMed] [Google Scholar]
- 49.Golombek DA, Agostino PV, Plano SA, Ferreyra GA. 2004. Signaling in the mammalian circadian clock: the NO/cGMP pathway. Neurochem. Int. 45, 929–936. ( 10.1016/j.neuint.2004.03.023) [DOI] [PubMed] [Google Scholar]
- 50.Morin LP, Allen CN. 2006. The circadian visual system, 2005. Brain Res. Rev. 51, 1–60. ( 10.1016/j.brainresrev.2005.08.003) [DOI] [PubMed] [Google Scholar]
- 51.Lowrey PL, Takahashi JS. 2000. Genetics of the mammalian circadian system: photic entrainment, circadian pacemaker mechanisms, and posttranslational regulation. Annu. Rev. Genet. 34, 533–562. ( 10.1146/annurev.genet.34.1.533) [DOI] [PubMed] [Google Scholar]
- 52.Agostino PV, Plano SA, Golombek DA. 2007. Sildenafil accelerates reentrainment of circadian rhythms after advancing light schedules. Proc. Natl Acad. Sci. USA 104, 9834–9839. ( 10.1073/pnas.0703388104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stephan FK. 2002. The ‘other’ circadian system: food as a Zeitgeber. J. Biol. Rhythms 17, 284–292. ( 10.1177/074873040201700402) [DOI] [PubMed] [Google Scholar]
- 54.Tataroglu O, Davidson AJ, Benvenuto LJ, Menaker M. 2006. The methamphetamine-sensitive circadian oscillator (MASCO) in mice. J. Biol. Rhythms 21, 185–194. ( 10.1177/0748730406287529) [DOI] [PubMed] [Google Scholar]
- 55.Mistlberger RE. 1994. Circadian food-anticipatory activity: formal models and physiological mechanisms. Neurosci. Biobehav. Rev. 18, 171–195. ( 10.1016/0149-7634(94)90023-X) [DOI] [PubMed] [Google Scholar]
- 56.Davidson AJ. 2009. Lesion studies targeting food-anticipatory activity. Eur. J. Neurosci. 30, 1658–1664. ( 10.1111/j.1460-9568.2009.06961.x) [DOI] [PubMed] [Google Scholar]
- 57.Masri S, Sassone-Corsi P. 2013. The circadian clock: a framework linking metabolism, epigenetics and neuronal function. Nat. Rev. Neurosci. 14, 69–75. ( 10.1038/nrn3393) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buijs RM, Scheer FA, Kreier F, Yi C, Bos N, Goncharuk VD, Kalsbeek A. 2006. Organization of circadian functions: interaction with the body. Prog. Brain Res. 153, 341–360. ( 10.1016/S0079-6123(06)53020-1) [DOI] [PubMed] [Google Scholar]
- 59.Bass J, Takahashi JS. 2010. Circadian integration of metabolism and energetics. Science 330, 1349–1354. ( 10.1126/science.1195027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J. 2007. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell. Metab. 6, 414–421. ( 10.1016/j.cmet.2007.09.006) [DOI] [PubMed] [Google Scholar]
- 61.Turek FW, et al. 2005. Obesity and metabolic syndrome in circadian clock mutant mice. Science 308, 1043–1045. ( 10.1126/science.1108750) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. 2009. Circadian timing of food intake contributes to weight gain. Obesity 17, 2100–2102. ( 10.1038/oby.2009.264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paschos GK, et al. 2012. Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat. Med. 18, 1768–1777. ( 10.1038/nm.2979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. 2009. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl Acad. Sci. USA 106, 4453–4458. ( 10.1073/pnas.0808180106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mansour HA, Wood J, Logue T, Chowdari KV, Dayal M, Kupfer DJ, Monk TH, Devlin B, Nimgaonkar VL. 2006. Association study of eight circadian genes with bipolar I disorder, schizoaffective disorder and schizophrenia. Genes Brain Behav. 5, 150–157. ( 10.1111/j.1601-183X.2005.00147.x) [DOI] [PubMed] [Google Scholar]
- 66.Nievergelt CM, et al. 2006. Suggestive evidence for association of the circadian genes PERIOD3 and ARNTL with bipolar disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 141B, 234–241. ( 10.1002/ajmg.b.30252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Partonen T, et al. 2007. Three circadian clock genes Per2, Arntl, and Npas2 contribute to winter depression. Ann. Med. 39, 229–238. ( 10.1080/07853890701278795) [DOI] [PubMed] [Google Scholar]
- 68.Benedetti F, Serretti A, Colombo C, Barbini B, Lorenzi C, Campori E, Smeraldi E. 2003. Influence of CLOCK gene polymorphism on circadian mood fluctuation and illness recurrence in bipolar depression. Am. J. Med. Genet. B Neuropsychiatr. Genet. 123B, 23–26. ( 10.1002/ajmg.b.20038) [DOI] [PubMed] [Google Scholar]
- 69.Soria V, et al. 2010. Differential association of circadian genes with mood disorders: CRY1 and NPAS2 are associated with unipolar major depression and CLOCK and VIP with bipolar disorder. Neuropsychopharmacology 35, 1279–1289. ( 10.1038/npp.2009.230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee KY, Song JY, Kim SH, Kim SC, Joo EJ, Ahn YM, Kim YS. 2010. Association between CLOCK 3111T/C and preferred circadian phase in Korean patients with bipolar disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 34, 1196–1201. ( 10.1016/j.pnpbp.2010.06.010) [DOI] [PubMed] [Google Scholar]
- 71.Kishi T, et al. 2009. CLOCK may predict the response to fluvoxamine treatment in Japanese major depressive disorder patients. Neuromolecular Med. 11, 53–57. ( 10.1007/s12017-009-8060-7) [DOI] [PubMed] [Google Scholar]
- 72.Sjöholm LK, Backlund L, Cheteh EH, Ek IR, Frisén L, Schalling M, Osby U, Lavebratt C, Nikamo P. 2010. CRY2 is associated with rapid cycling in bipolar disorder patients. PLoS ONE 5, e12632 ( 10.1371/journal.pone.0012632) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lavebratt C, et al. 2010. CRY2 is associated with depression. PLoS ONE 5, e9407 ( 10.1371/journal.pone.0009407) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johansson C, et al. 2003. Circadian clock-related polymorphisms in seasonal affective disorder and their relevance to diurnal preference. Neuropsychopharmacology 28, 734–739. ( 10.1038/sj.npp.1300121) [DOI] [PubMed] [Google Scholar]
- 75.Kripke DF, Nievergelt CM, Joo E, Shekhtman T, Kelsoe JR. 2009. Circadian polymorphisms associated with affective disorders. J. Circadian Rhythms 7, 2 ( 10.1186/1740-3391-7-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lavebratt C, Sjöholm LK, Partonen T, Schalling M, Forsell Y. 2010. PER2 variantion is associated with depression vulnerability. Am. J. Med. Genet. B Neuropsychiatr. Genet. 153B, 570–581. ( 10.1002/ajmg.b.31021) [DOI] [PubMed] [Google Scholar]
- 77.Benedetti F, Dallaspezia S, Colombo C, Pirovano A, Marino E, Smeraldi E. 2008. A length polymorphism in the circadian clock gene Per3 influences age at onset of bipolar disorder. Neurosci. Lett. 445, 184–187. ( 10.1016/j.neulet.2008.09.002) [DOI] [PubMed] [Google Scholar]
- 78.McGrath CL, et al. 2009. Evidence for genetic association of RORB with bipolar disorder. BMC Psychiatry 9, 70 ( 10.1186/1471-244X-9-70) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Andretic R, Chaney S, Hirsh J. 1999. Requirement of circadian genes for cocaine sensitization in Drosophila. Science 285, 1066–1068. ( 10.1126/science.285.5430.1066) [DOI] [PubMed] [Google Scholar]
- 80.Roybal K, et al. 2007. Mania-like behavior induced by disruption of CLOCK. Proc. Natl Acad. Sci. USA 104, 6406–6411. ( 10.1073/pnas.0609625104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Masana MI, Sumaya IC, Becker-Andre M, Dubocovich ML. 2007. Behavioral characterization and modulation of circadian rhythms by light and melatonin in C3H/HeN mice homozygous for the RORbeta knockout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R2357–R2367. ( 10.1152/ajpregu.00687.2006) [DOI] [PubMed] [Google Scholar]
- 82.Williams SR, Zies D, Mullegama SV, Grotewiel MS, Elsea SH. 2012. Smith–Magenis syndrome results in disruption of CLOCK gene transcription and reveals an integral role for RAI1 in the maintenance of circadian rhythmicity. Am. J. Hum. Genet. 90, 941–949. ( 10.1016/j.ajhg.2012.04.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dupuis J, et al. 2010. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat. Genet. 42, 105–116. ( 10.1038/ng.520) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sakai T, Tamura T, Kitamoto T, Kidokoro Y. 2004. A clock gene, period, plays a key role in long-term memory formation in Drosophila. Proc. Natl Acad. Sci. USA 101, 16 058–16 063. ( 10.1073/pnas.0401472101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Van der Zee EA, Havekes R, Barf RP, Hut RA, Nijholt IM, Jacobs EH, Gerkema MP. 2008. Circadian time-place learning in mice depends on Cry genes. Curr. Biol. 18, 844–848. ( 10.1016/j.cub.2008.04.077) [DOI] [PubMed] [Google Scholar]
- 86.Abarca C, Albrecht U, Spanagel R. 2002. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc. Natl Acad. Sci. USA 99, 9026–9030. ( 10.1073/pnas.142039099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, Nestler EJ. 2005. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc. Natl Acad. Sci. USA 102, 9377–9381. ( 10.1073/pnas.0503584102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dong L, et al. 2011. Effects of the circadian rhythm gene period 1 (Per1) on psychosocial stress-induced alcohol drinking. Am. J. Psychiatry 168, 1090–1098. ( 10.1176/appi.ajp.2011.10111579) [DOI] [PubMed] [Google Scholar]
- 89.Spanagel R, et al. 2005. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat. Med. 11, 35–42. ( 10.1038/nm1163) [DOI] [PubMed] [Google Scholar]
- 90.Doi M, Yujnovsky I, Hirayama J, Malerba M, Tirotta E, Sassone-Corsi P, Borrelli E. 2006. Impaired light masking in dopamine D2 receptor-null mice. Nat. Neurosci. 9, 732–734. ( 10.1038/nn1711) [DOI] [PubMed] [Google Scholar]
- 91.Hood S, Cassidy P, Cossette MP, Weigl Y, Verwey M, Robinson B, Stewart J, Amir S. 2010. Endogenous dopamine regulates the rhythm of expression of the clock protein PER2 in the rat dorsal striatum via daily activation of D2 dopamine receptors . J. Neurosci. 30, 14 046–14 058. ( 10.1523/JNEUROSCI.2128-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yujnovsky I, Hirayama J, Doi M, Borrelli E, Sassone-Corsi P. 2006. Signaling mediated by the dopamine D2 receptor potentiates circadian regulation by CLOCK:BMAL1. Proc. Natl Acad. Sci. USA 103, 6386–6391. ( 10.1073/pnas.0510691103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McIntyre RS. 2009. Managing weight gain in patients with severe mental illness. J. Clin. Psychiatry 70, e23 ( 10.4088/JCP.7075cc4c) [DOI] [PubMed] [Google Scholar]
- 94.Scott AJ. 2000. Shift work and health. Prim. Care 27, 1057–1079. ( 10.1016/S0095-4543(05)70189-5) [DOI] [PubMed] [Google Scholar]
- 95.Hasler BP, Buysse DJ, Kupfer DJ, Germain A. 2010. Phase relationships between core body temperature, melatonin, and sleep are associated with depression severity: further evidence for circadian misalignment in non-seasonal depression. Psychiatry Res. 178, 205–207. ( 10.1016/j.psychres.2010.04.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Frank E, Swartz HA, Kupfer DJ. 2000. Interpersonal and social rhythm therapy: managing the chaos of bipolar disorder. Biol. Psychiatry 48, 593–604. ( 10.1016/S0006-3223(00)00969-0) [DOI] [PubMed] [Google Scholar]
- 97.Hlastala SA, Frank E. 2006. Adapting interpersonal and social rhythm therapy to the developmental needs of adolescents with bipolar disorder. Dev. Psychopathol. 18, 1267–1288. ( 10.1017/S0954579406060615) [DOI] [PubMed] [Google Scholar]
- 98.Magnusson A, Boivin D. 2003. Seasonal affective disorder: an overview. Chronobiol. Int. 20, 189–207. ( 10.1081/CBI-120019310) [DOI] [PubMed] [Google Scholar]
- 99.Monteleone P, Martiadis V, Maj M. 2011. Circadian rhythms and treatment implications in depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 1569–1574. ( 10.1016/j.pnpbp.2010.07.028) [DOI] [PubMed] [Google Scholar]
- 100.Terman M, Terman JS. 2005. Light therapy for seasonal and nonseasonal depression: efficacy, protocol, safety, and side effects. CNS Spectr. 10, 647–663; quiz 672. [DOI] [PubMed] [Google Scholar]
- 101.McCarthy MJ, Welsh DK. 2012. Cellular circadian clocks in mood disorders. J. Biol. Rhythms 27, 339–352. ( 10.1177/0748730412456367) [DOI] [PubMed] [Google Scholar]
- 102.Daan S. 2000. Learning and circadian behavior. J. Biol. Rhythms 15, 296–299. ( 10.1177/074873000129001396) [DOI] [PubMed] [Google Scholar]
- 103.Eckel-Mahan KL, Storm DR. 2009. Circadian rhythms and memory: not so simple as cogs and gears. EMBO Rep. 10, 584–591. ( 10.1038/embor.2009.123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gerstner JR, Yin JC. 2010. Circadian rhythms and memory formation. Nat. Rev. Neurosci. 11, 577–588. ( 10.1038/nrn2881) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gritton HJ, Kantorowski A, Sarter M, Lee TM. 2012. Bidirectional interactions between circadian entrainment and cognitive performance. Learn. Mem. 19, 126–141. ( 10.1101/lm.023499.111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Devan BD, et al. 2001. Circadian phase-shifted rats show normal acquisition but impaired long-term retention of place information in the water task. Neurobiol. Learn. Mem. 75, 51–62. ( 10.1006/nlme.1999.3957) [DOI] [PubMed] [Google Scholar]
- 107.Gibson EM, Wang C, Tjho S, Khattar N, Kriegsfeld LJ. 2010. Experimental ‘jet lag’ inhibits adult neurogenesis and produces long-term cognitive deficits in female hamsters . PLoS ONE 5, e15267 ( 10.1371/journal.pone.0015267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Loh DH, Navarro J, Hagopian A, Wang LM, Deboer T, Colwell CS. 2010. Rapid changes in the light/dark cycle disrupt memory of conditioned fear in mice. PLoS ONE 5, e12546 ( 10.1371/journal.pone.0012546) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Folkard S. 1996. Effects on performance efficiency. In Shiftwork: problems and solution (eds Coquhoun WP, Costa G, Folkard S, Knauth P.), pp. 65–87. Frankfurt, Germany: Peter Lang. [Google Scholar]
- 110.Cho K, Ennaceur A, Cole JC, Suh CK. 2000. Chronic jet lag produces cognitive deficits. J. Neurosci. 20, RC66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cho K. 2001. Chronic ‘jet lag’ produces temporal lobe atrophy and spatial cognitive deficits. Nat. Neurosci. 4, 567–568. ( 10.1038/88384) [DOI] [PubMed] [Google Scholar]
- 112.Santhi N, Horowitz TS, Duffy JF, Czeisler CA. 2007. Acute sleep deprivation and circadian misalignment associated with transition onto the first night of work impairs visual selective attention. PLoS ONE 2, e1233 ( 10.1371/journal.pone.0001233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wright KP, Jr, Hull JT, Czeisler CA. 2002. Relationship between alertness, performance, and body temperature in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 283, R1370–R1377. [DOI] [PubMed] [Google Scholar]
- 114.Kyriacou CP, Hastings MH. 2010. Circadian clocks: genes, sleep, and cognition. Trends Cogn. Sci. 14, 259–267. ( 10.1016/j.tics.2010.03.007) [DOI] [PubMed] [Google Scholar]
- 115.Drew MR, Simpson EH, Kellendonk C, Herzberg WG, Lipatova O, Fairhurst S, Kandel ER, Malapani C, Balsam PD. 2007. Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing. J. Neurosci. 27, 7731–7739. ( 10.1523/JNEUROSCI.1736-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cheng RK, MacDonald CJ, Meck WH. 2006. Differential effects of cocaine and ketamine on time estimation: implications for neurobiological models of interval timing. Pharm. Biochem. Behav. 85, 114–122. ( 10.1016/j.pbb.2006.07.019) [DOI] [PubMed] [Google Scholar]
- 117.Coull JT, Cheng RK, Meck WH. 2011. Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacology 36, 3–25. ( 10.1038/npp.2010.113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wiener M, Lohoff FW, Coslett HB. 2011. Double dissociation of dopamine genes and timing in humans. J. Cogn. Neurosci. 23, 2811–2821. ( 10.1162/jocn.2011.21626) [DOI] [PubMed] [Google Scholar]
- 119.Balcı F, Wiener M, Cavdaroğlu B, Branch Coslett H. 2013. Epistasis effects of dopamine genes on interval timing and reward magnitude in humans. Neuropsychologia 51, 293–308. ( 10.1016/j.neuropsychologia.2012.08.002) [DOI] [PubMed] [Google Scholar]
- 120.Meck WH, Cheng RK, MacDonald CJ, Gainetdinov RR, Caron MG, Cevik MÖ. 2012. Gene-dose dependent effects of methamphetamine on interval timing in dopamine-transporter knockout mice. Neuropharmacology 62, 1221–1229. ( 10.1016/j.neuropharm.2011.01.042) [DOI] [PubMed] [Google Scholar]
- 121.Cheng RK, Meck WH, Williams CL. 2006. Alpha7 nicotinic acetylcholine receptors and temporal memory: synergistic effects of combining prenatal choline and nicotine on reinforcement-induced resetting of an interval clock. Pharmacol. Biochem. Behav. 85, 114–122. ( 10.1101/lm.31506) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Droit-Volet S, Meck WH. 2007. How emotions colour our perception of time. Trends Cogn. Sci. 11, 504–513. ( 10.1016/j.tics.2007.09.008) [DOI] [PubMed] [Google Scholar]
- 123.Aschoff J. 1985. On the perception of time during prolonged temporal isolation. Hum. Neurobiol. 4, 41–52. [PubMed] [Google Scholar]
- 124.Chandrashekaran MΚ, Marimuthu G, Subbaraj R, Kumarasamy P, Ramkumar MS, Sripathi K. 1991. Direct correlation between the circadian sleep–wakefulness rhythm and time estimation in humans under social and temporal isolation. J. Biosci. 16, 97–101. ( 10.1007/BF02703361) [DOI] [Google Scholar]
- 125.Pati AK, Gupta S. 1994. Time estimation circadian rhythm in shift workers and diurnally active humans. J. Biosci. 19, 325–330. ( 10.1007/BF02716822) [DOI] [Google Scholar]
- 126.Meck WH. 1991. Modality-specific circadian rhythmicities influence mechanisms of attention and memory for interval timing. Learn. Motiv. 22, 153–179. ( 10.1016/0023-9690(91)90021-Y) [DOI] [Google Scholar]
- 127.Kuriyama K, et al. 2005. Diurnal fluctuation of time perception under 30-h sustained wakefulness. Neurosci. Res. 53, 123–128. ( 10.1016/j.neures.2005.06.006) [DOI] [PubMed] [Google Scholar]
- 128.Lustig C, Meck WH. 2001. Paying attention to time as one gets older. Psychol. Sci. 12, 478–484. ( 10.1111/1467-9280.00389) [DOI] [PubMed] [Google Scholar]
- 129.Shurtleff D, Raslear TG, Simmons L. 1990. Circadian variations in time perception in rats. Physiol. Behav. 47, 931–939. ( 10.1016/0031-9384(90)90021-U) [DOI] [PubMed] [Google Scholar]
- 130.Soshi T, Kuriyama K, Aritake S, Enomoto M, Hida A, Tamura M, Kim Y, Mishima K. 2010. Sleep deprivation influences diurnal variation of human time perception with prefrontal activity change: a functional near-infrared spectroscopy study. PLoS ONE 5, e8395 ( 10.1371/journal.pone.0008395) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kyriacou CP, Hall J. 1980. Circadian rhythm mutations in Drosophila melanogaster affect short-term fluctuations in the male's courtship song. Proc. Natl Acad. Sci. USA 77, 6729–6733. ( 10.1073/pnas.77.11.6729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Agostino PV, do Nascimento M, Bussi IL, Eguía MC, Golombek DA. 2011. Circadian modulation of interval timing in mice. Brain Res. 1370, 154–163. ( 10.1016/j.brainres.2010.11.029) [DOI] [PubMed] [Google Scholar]
- 133.Li SX, Liu LJ, Jiang WG, Lu L. 2009. Morphine withdrawal produces circadian rhythm alterations of clock genes in mesolimbic brain areas and peripheral blood mononuclear cells in rats. J. Neurochem. 109, 1668–1679. ( 10.1111/j.1471-4159.2009.06086.x) [DOI] [PubMed] [Google Scholar]
- 134.Natsubori A, Honma K, Honma S. 2013. Differential responses of circadian Per2 expression rhythms in discrete brain areas to daily injection of methamphetamine and restricted feeding in rats. Eur. J. Neurosci. 37, 251–258. ( 10.1111/ejn.12034) [DOI] [PubMed] [Google Scholar]
- 135.Yoon SO, Chikaraishi DM. 1992. Tissue-specific transcription of the rat tyrosine hydroxylase gene requires synergy between an AP-1 motif and an overlapping E box-containing dyad. Neuron 9, 55–67. ( 10.1016/0896-6273(92)90220-8) [DOI] [PubMed] [Google Scholar]
- 136.Kawarai T, Kawakami H, Yamamura Y, Nakamura S. 1997. Structure and organization of the gene encoding human dopamine transporter. Gene 195, 11–18. ( 10.1016/S0378-1119(97)00131-5) [DOI] [PubMed] [Google Scholar]
- 137.Castaneda TR, de Prado BM, Prieto D, Mora F. 2004. Circadian rhythms of dopamine, glutamate and GABA in the striatum and nucleus accumbens of the awake rat: modulation by light. J. Pineal Res. 36, 177–185. ( 10.1046/j.1600-079X.2003.00114.x) [DOI] [PubMed] [Google Scholar]
- 138.Sleipness EP, Sorg BA, Jansen HT. 2007. Diurnal differences in dopamine transporter and tyrosine hydroxylase levels in rat brain: dependence on the suprachiasmatic nucleus. Brain Res. 1129, 34–42. ( 10.1016/j.brainres.2006.10.063) [DOI] [PubMed] [Google Scholar]
- 139.Ikeda E, Matsunaga N, Kakimoto K, Hamamura K, Hayashi A, Koyanagi S, Ohdo S. 2013. Molecular mechanism regulating 24-hour rhythm of dopamine D3 receptor expression in mouse ventral striatum. Mol. Pharmacol. 83, 959–967. ( 10.1124/mol.112.083535) [DOI] [PubMed] [Google Scholar]
- 140.Shumay E, Fowler JS, Wang GJ, Logan J, Alia-Klein N, Goldstein RZ, Maloney T, Wong C, Volkow ND. 2012. Repeat variation in the human PER2 gene as a new genetic marker associated with cocaine addiction and brain dopamine D2 receptor availability. Transl. Psychiatry 2, e86 ( 10.1038/tp.2012.11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Imbesi M, Yildiz S, Dirim Arslan A, Sharma R, Manev H, Uz T. 2009. Dopamine receptor-mediated regulation of neuronal ‘clock’ gene expression. Neuroscience 158, 537–544. ( 10.1016/j.neuroscience.2008.10.044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Oster H, Maronde E, Albrecht U. 2002. The circadian clock as a molecular calendar. Chronobiol. Int. 19, 507–516. ( 10.1081/CBI-120004210) [DOI] [PubMed] [Google Scholar]
- 143.Ikegami K, Yoshimura T. 2012. Circadian clocks and the measurement of daylength in seasonal reproduction. Mol. Cell Endocrinol. 349, 76–81. ( 10.1016/j.mce.2011.06.040) [DOI] [PubMed] [Google Scholar]