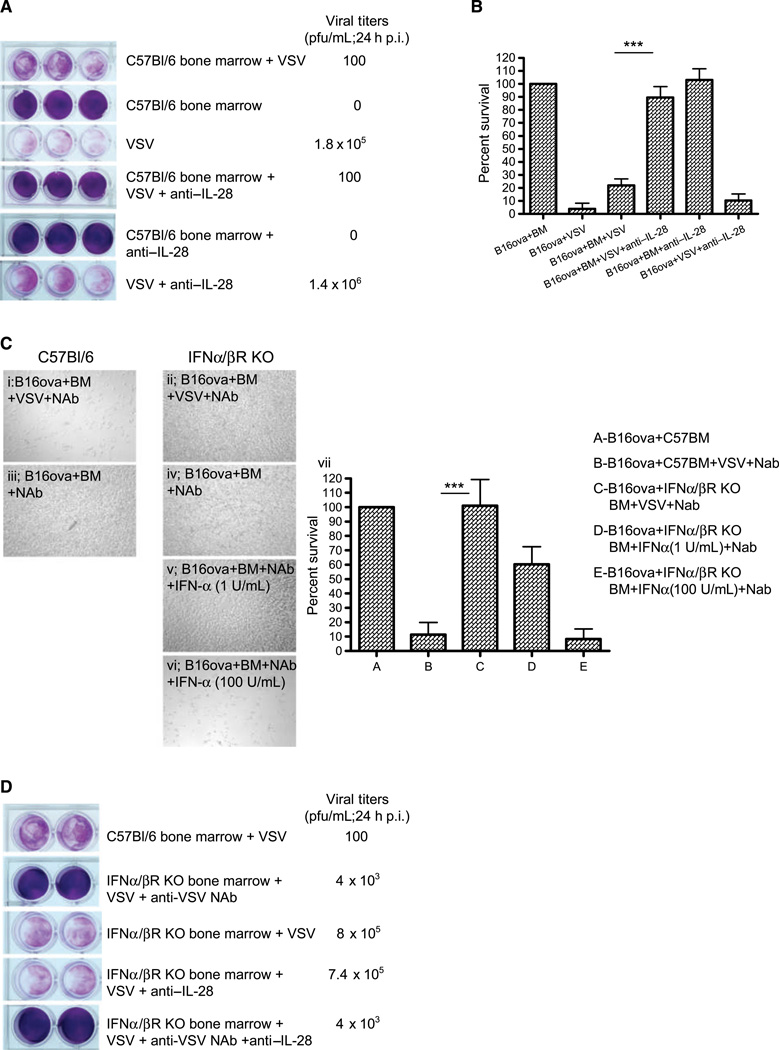
Figure 3.
VSV-activated bone marrow cytotoxicity depends upon type I and III IFNs. A, B16ova cells were cocultured with bone marrow cells as in Fig. 1A, with or without anti-IL-28 antibody (15 ug/mL) upon infection with VSV. Twenty-four hours later, supernatants were harvested for viral titer and cell viability was assessed by crystal violet staining (A) and MTT assay (B). C, light microscopy of cocultures of B16ova cells with either C57Bl/6-derived bone marrow (i and iii) or bone marrow from IFN-α/βR KO mice (ii, iv, v, and vi) 48 h following treatment with VSV (MOI 0.1) +NAb (I and ii) or bone marrow+Nab (no VSV; iii and iv), or with bone marrow from IFN-α/βR KO mice +Nab (no VSV) with added IFN-α at 1 (v) or 100 (vi) U/mL. MTT assay of these results is shown in vii. D, the experiment in A was repeated with coculture of B16ova with C57Bl/6- or IFN-α/βR KO-derived bone marrow, with VSV +/− anti-IL-28 antibody. Twenty-four hours after VSV, supernatants were harvested for viral titer and cell viability was assessed by crystal violet. ***, P < 0.001.