Table 1.
Preliminary Results of Allylic Substitution Reactionsa
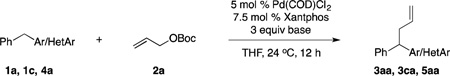 | ||||||
|---|---|---|---|---|---|---|
| Entry | Pronuelephiles | Base | Product | Yieldb (%) | ||
| 1d | 1a | LiN(SiMe3)2 | 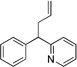 |
3aa | 80 | |
| 2d | 1a | NaN(SiMe3)2 | 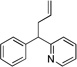 |
3aa | (99c) | |
| 3 | 1a | NaN(SiMe3)2 | 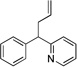 |
3aa | (99c) | |
| 4 | 1c | NaN(SiMe3)2 | 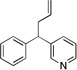 |
3ca | 27 | |
| 5 | 1c | KN(SiMe3)2 | 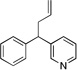 |
3ca | 70 | |
| 6 | 4a | KN(SiMe3)2 | 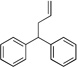 |
5aa | 10 | |
Reaction conducted on a 0.1 mmol scale with 1 equiv of pronucleophile and 2 equiv of 2a at 0.1 M.
Yield determined by 1H NMR spectroscopy of the crude reaction mixture.
Isolated yield after chromatographic purification.
1 equiv of NEt3.
