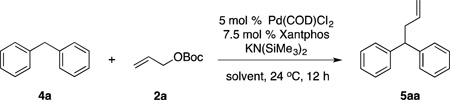
Table 2.
Optimization of Allylic Substitution with Diphenyl-methane 4aa
 | |||
|---|---|---|---|
| Entry | Ratio (4a:base:2a) |
Solvent | yieldb(%) |
| 1 | 1:3:2 | THF | 10 |
| 2 | 1:3:2 | DME | 55 |
| 3 | 1:3:3 | DME | 79 |
| 4 | 1:4:3 | DME | 88 |
| 5 | 1:5:3 | DME | (95c) |
| 6 | 1:5:2 | DME | 62 |
| 7d | 1:5:3 | DME | 68 |
Reaction conducted on a 0.1 mmol scale at 0.1 M.
Yield determined by 1H NMR spectroscopy of the crude reaction mixture.
Isolated yield after chromatographic purification.
2.5 mol % Pd(COD)Cl2/3.75 mol % Xantphos.
