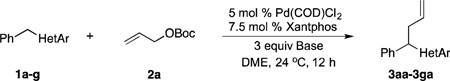
Table 3.
Scope of Heterocyclic Diarylmethanes in the Allylic Substitutiona
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Pronucleophlles | Base | Products | Yieldb (%) | ||
| 1c | 1a | NaN(SiMe3)2 | 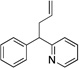 |
3aa | 99 | |
| 2c | 1b | LiN(SiMe3)2 | 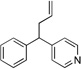 |
3ba | 99 | |
| 3 | 1c | KN(SiMe3)2 | 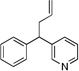 |
3ca | 91 | |
| 4 | 1d | NaN(SiMe3)2 | 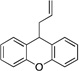 |
3da | 82 | |
| 5d | 1e | NaN(SiMe3)2 |  |
3ea | 80 | |
| 6 | 1f | NaN(SiMe3)2 | 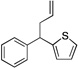 |
3fa | 93 | |
| 7 | 1g | LiN(SiMe3)2 | 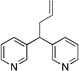 |
3ga | 85 | |
Reaction conducted on a 0.1 mmol scale with 1 equiv of 1 and 2 equiv of 2a at 0.1 M .
Isolated yield after chromatographic purification.
1 mol % Pd(COD)Cl2, 1.5 mol % Xantphos.
2.5 equiv of NaN(SiMe3)2 and 2.5 equiv of 15-crown-5.
