Table 4.
Scope of Diphenylmethane Derivatives in Allylic Substitution Reactionsa
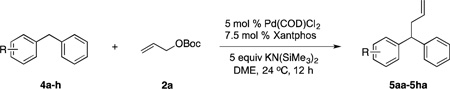 | |||||
|---|---|---|---|---|---|
| Entry | Pronucleophlles | Products | Yieldb(%) | ||
| 1 | 4a | 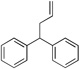 |
5aa | 95 | |
| 2 | 4b | 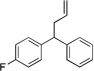 |
5ba | 84 | |
| 3 | 4c | 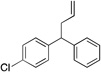 |
5ca | 95 | |
| 4 | 4d | 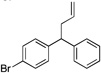 |
5da | 73 | |
| 5c | 4e | 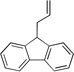 |
5ea | 87 | |
| 6d | 4f | 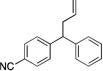 |
5fa | 90 | |
| 7e, g | 4g | 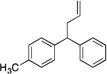 |
5ga | 68 | |
| 8f, g | 4h | 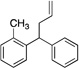 |
5ha | 70 | |
Reaction conducted on a 0.1 mmol scale with 1 equiv of 4 and 3 equiv of 2a at 0.1 M.
Isolated yield after chromatographic purification.
1.5 equiv of LiN(SiMe3)2 and 1.1 equiv of 2a.
1.5 equiv of KN(SiMe3)2 and 2 equiv of 2a.
8 equiv of KN(SiMe3)2.
10 equiv of KN(SiMe3)2.
Reaction conducted at 50 °C.
