Table 6.
Scope of Electrophiles in Allylic Substitution Reactionsa
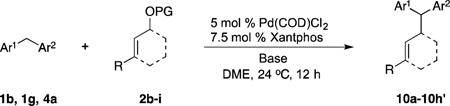 | ||||||
|---|---|---|---|---|---|---|
| Entry | Electrophiles | Pronueleophlies | Products | Yieldb (%) | ||
| 1c | PG = Boc | 2b | 1g | 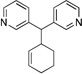 |
10a | 89 |
| 2c | PG = Bz | 2c | 1g | 90 | ||
| 3d | 2d | 1g | 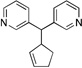 |
10b | 90 | |
| 4e | 2e | 1g | 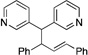 |
10c | 87 | |
| (tranc: cis = 10:1)i | ||||||
| 5f | PG = Boc | 2b | 4a | 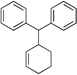 |
10d | 85 |
| 6f | PG = Bz | 2c | 4a | 20 | ||
| 7f | PG = Piv | 2f | 4a | 70 | ||
| 8f, g | 2d | 4a | 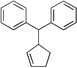 |
10e | 94 | |
| 9e, h | 2g | 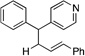 |
10f | 91 (L:B=2.6:1)i |
||
| 1b | ||||||
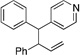 |
10f’ | |||||
| 10e, h | 2h | 1b | 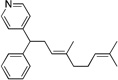 |
10g | 88 (L:B=1.9:1)i |
|
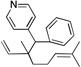 |
10g’ | |||||
| 11e, h | 2i | 1b | 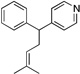 |
10h | 93 (L:B=1:4.5)i |
|
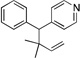 |
10h’ | |||||
Reaction conducted on a 0.1 mmol scale at 0.1 M.
Isolated yield after chromatographic purification.
3 equiv of NaN(SiMe3)2 and 2 equiv of 2.
3 equiv of KN(SiMe3)2 and 2 equiv of 2.
3 equiv of LiN(SiMe3)2 and 2 equiv of 2.
5 equiv of KN(SiMe3)2 and 3 equiv of 2.
Reaction conducted at 50 °C.
Reaction conducted at 0 °C.
Ratio of trans:cis or line-ar:branched (L:B) determined by 1H NMR.
