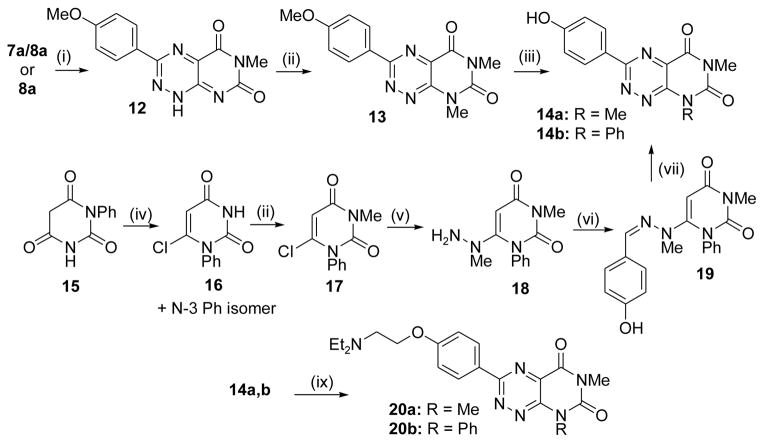
Scheme 2.
Synthesis of analogues in the fervenulin series. (i) DMF, 90 °C (65 – 73% yield); (ii) for 12 and 16, dimethyl sulfate, Cs2CO3, acetone, rt - 50 °C (84 – 97% yield); (iii) for 14a, BBr3, DCM (65% yield); (iv) POCl3 (48% yield); (v) methylhydrazine, EtOH, reflux (88% yield); (vi) 4-OH-benzaldehyde, EtOH, reflux (64% yield); (vii) NaNO2, aq. AcOH, 0 °C – 25 °C, then DTT, EtOH (44% yield); (viii) ClCH2CH2NEt2. HCl, Cs2CO3, acetone, rt - 50 °C (44 – 63% yield). See Supplemental Materials for experimental details.