Table 1.
Initial Optimization of Hydroarylation Conditions[a]
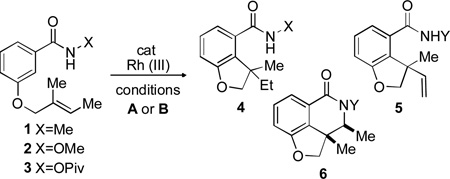 | |||||
|---|---|---|---|---|---|
| entry | X | Y | condition | 4:5:6 | Conv.[b] |
| 1 | Me | Me | A, 17 h | 100:0:0 | 100 |
| 2 | OMe | H | A, 17 h | 71:29:0 | 53 |
| 3 | OPiv | H | A, 17 h | 0:0:100 | 61[c] |
| 4 | OMe | H | B, 2 h | 0:100:0 | 100 |
| 5 | OPiv | H | B, 12 h | 0:22:78 | 100 |
Reactions conducted on 0.1 mmol scale. A: [RhCp*(MeCN)3](SbF6)2 (1 mol%), 1 equiv. tBuCO2H, 1,2-dichloroethane (0.2 M), 80 °C; B: [RhCp*Cl2]2 (2.5 mol%), CsOAc (2 equiv.), MeOH (0.2 M), rt
Conversion determined by 1H NMR.
Starting material hydrolysis also observed.
