Table 11.
Examples of 1,2,4-trioxolanes produced by the ozonolysis of alkenes.
| Alkene 163 | Ozonolysis conditions | 1,2,4-Trioxolane 165 | Yield, % | Reference |
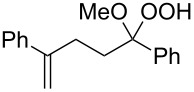 |
Et2O, −70 °C | 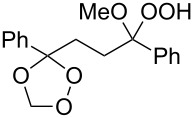 |
24 | [270] |
 |
Et2O, −70 °C | 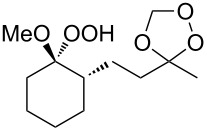 |
27 | [270] |
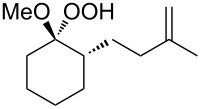
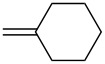 |
hexane, −78 °C | 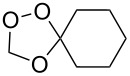 |
78 | [256] |
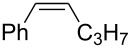 |
hexane, −78 °C | 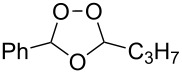 |
73 | [256] |
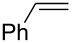 |
hexane, −78 °C | 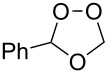 |
77 | [256] |
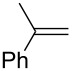 |
hexane, −78 °C | 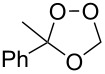 |
61 | [256] |
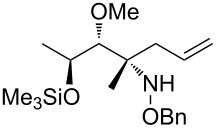 |
isooctane/CCl4, −78 °C, 1 h | 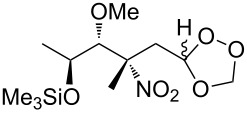 |
>82 | [271] |
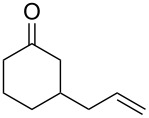 |
CH2Cl2, −78 °C | 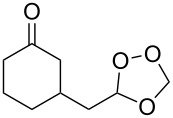 |
95 | [272] |
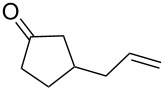 |
CH2Cl2, −78 °C | 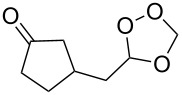 |
90 | [272] |
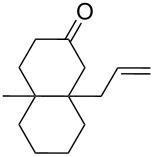 |
CH2Cl2, −78 °C | 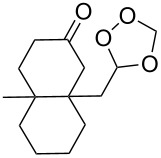 |
92 | [272] |
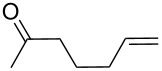
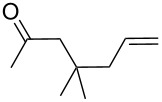 |
CH2Cl2, −78 °C | 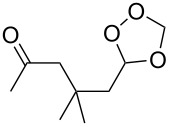 |
93 | [272] |
 |
CH2Cl2, −78 °C | 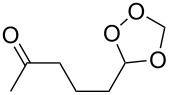 |
93 | [272] |
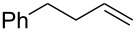 |
CH2Cl2, −78 °C | 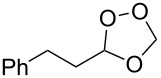 |
94 | [272] |
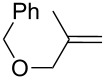 |
pentane, −78 °C |
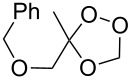 |
63 | [272] |
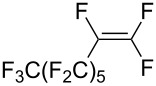 |
freon-113, 15–20 °C, 2 h | 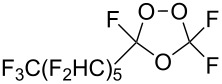 |
The yield was not determined | [273–274] |
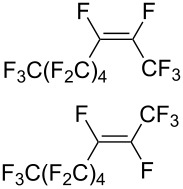 |
freon-113, 15–20 °C, 2 h | 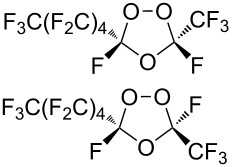 |
The yield was not determined | [273] |
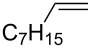 |
CH2Cl2, −78 °C | 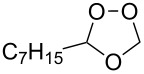 |
96 | [275] |
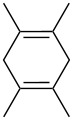 |
polymer-based, −78 °C, 8 h | 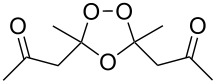 |
23 | [276] |
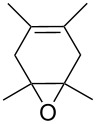 |
polymer-based, −78 °C, 3 h | 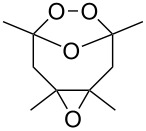 |
38 | [276] |
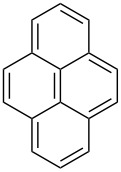 |
CH2Cl2, −70 °C | 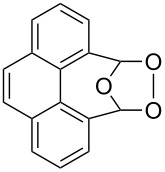 |
48 | [277] |
 |
without solvent, −133 to −43 °C | 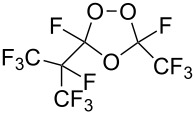 |
100 | [278] |
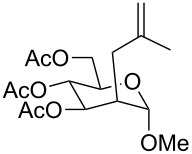 |
1) CH2Cl2, −78 °C, 15 min. 2) Me2S, rt, 6 h |
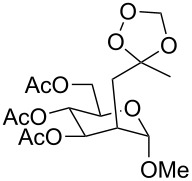 |
71 | [279] |
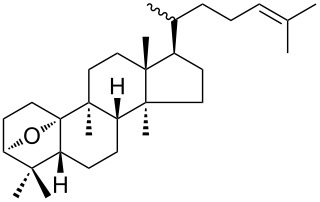 |
hexane, −78 °C, 30 min |
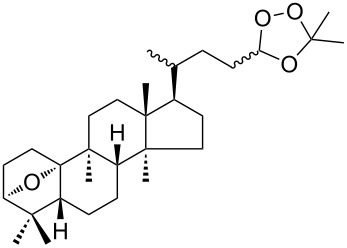 |
6 | [280] |
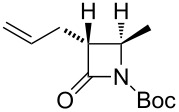 |
CH2Cl2, −78 °C, 20 min | 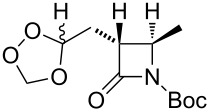 |
The yield was not determined | [281] |
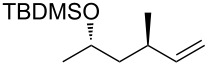 |
CH2Cl2, −78 °C, 2 h | 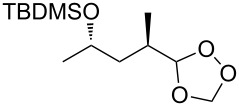 |
>97 | [282] |
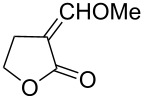 |
CDCl3, −65 °C | 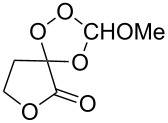 |
88 | [283] |
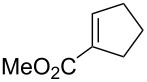 |
CFCl3, −70 °C | 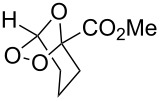 |
100 | [283] |
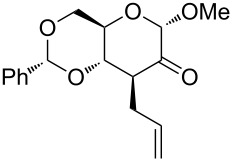 |
CH2Cl2, −78 °C, 1 h | 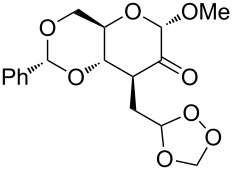 |
85 | [284] |
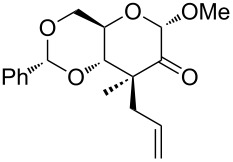 |
CH2Cl2, −78 °C, 1 h | 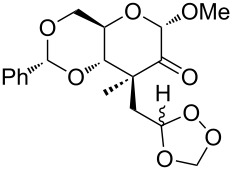 |
70 | [285] |
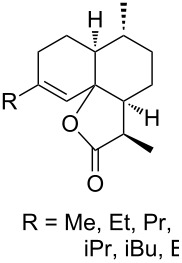 |
pentane, −78 °C |
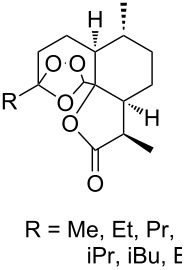 |
10-30 | [148–152] |
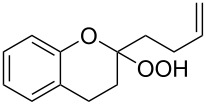 |
Et2O/CH3OH, −78 °C | 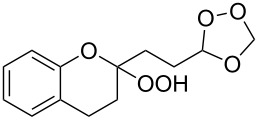 |
12 | [254] |
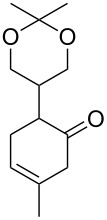 |
CH2Cl2, −78 °C | 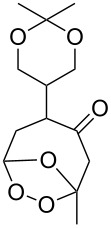 |
92 | [286] |
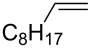 |
CH2Cl2, 0 °C | 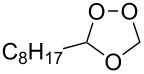 |
95 | [287–288] |
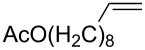 |
H2O/CH2Cl2, 0 °C |
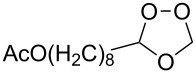 |
72 | [289] |
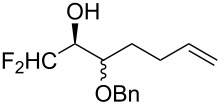 |
1) SiO2, −78 °C; 2) Me2S, MeOH |
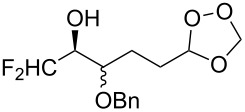 |
30 | [290] |
