Table 13.
Examples of ozonides (1,2,4-trioxolanes) synthesized by the Griesbaum method.
| Oxime 174 | Ketone 175 | Ozonolysis conditions | 1,2,4-Trioxolane 176 | Yield, % | Ref. |
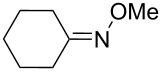 |
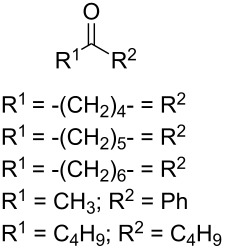 |
hexane, −78 °C | 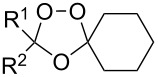 |
47–67 | [256] |
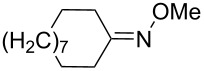 |
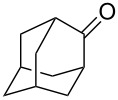 |
pentane, CH2Cl2, 0 °C | 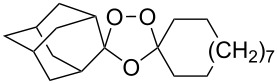 |
54 | [91] |
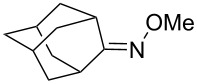 |
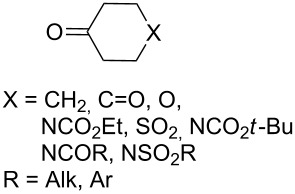 |
pentane, CH2Cl2, 0 °C | 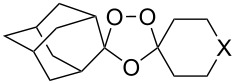 |
10–75 | [91,94] [95,296] |
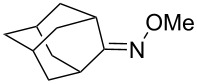 |
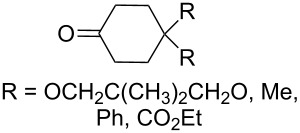 |
pentane, CH2Cl2, 0 °C | 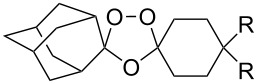 |
23–50 | [91–93] |
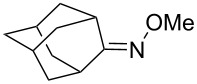 |
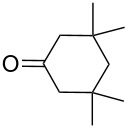 |
pentane, 0 °C | 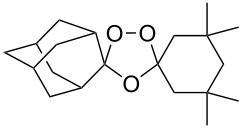 |
48 | [92–93] |
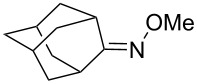 |
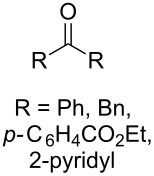 |
pentane, CH2Cl2, 0 °C | 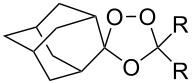 |
32–58 | [91–93] |
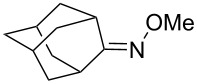 |
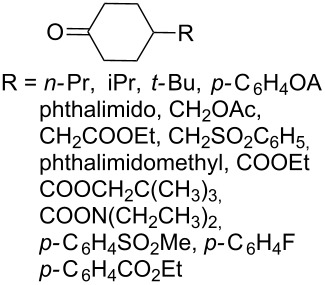 |
pentane, CH2Cl2, 0 °C | 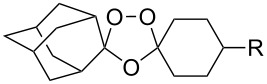 |
20–70 | [91–93,96] [97,297] |
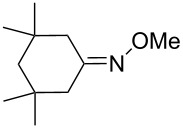 |
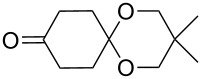 |
pentane, 0 °C | 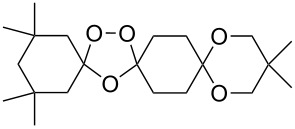 |
38 | [91] |
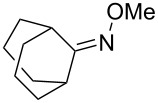 |
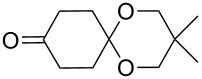 |
pentane, 0 °C | 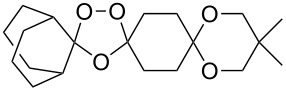 |
41 | [91] |
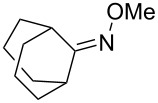 |
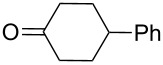 |
pentane, CH2Cl2, 0 °C | 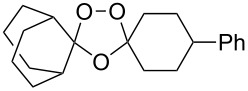 |
33 | [91] |
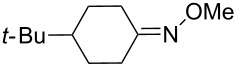 |
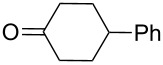 |
hexane, CH2Cl2, 0 °C |
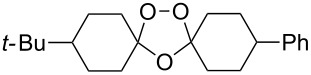 |
17 | [91] |
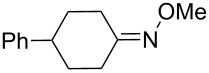 |
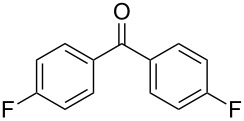 |
pentane, CH2Cl2, 0 °C | 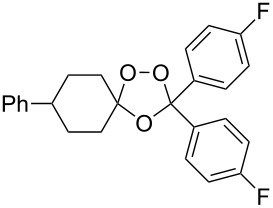 |
27 | [91] |
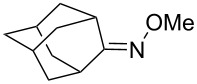 |
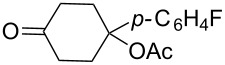 |
pentane, CH2Cl2, 0 °C | 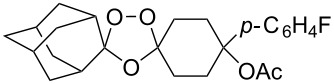 |
53 | [92–93] |
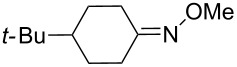 |
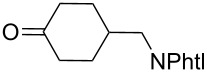 |
pentane, CH2Cl2, 0 °C | 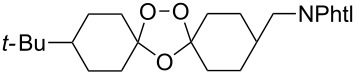 |
n.d.a | [96–97] |
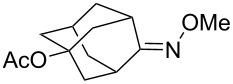 |
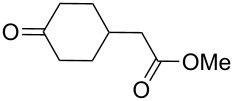 |
cyclohexane CH2Cl2, 0 °C | 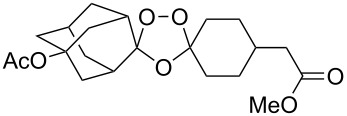 |
30 | [298] |
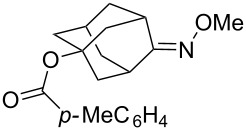 |
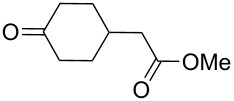 |
cyclohexane CH2Cl2, 0 °C | 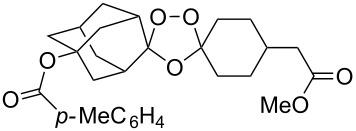 |
54 | [298] |
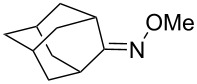 |
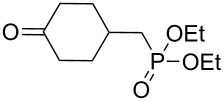 |
cyclohexane, CH2Cl2, 0 °C | 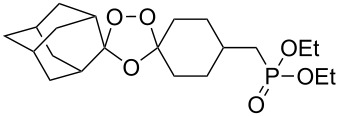 |
78 | [258] |
aYield was not determined
