Table 22.
Examples of 1,2,4-trioxanes synthesized through the Isayama–Mukaiyama peroxysilylation.
| Unsaturated alcohol 352 | Carbonyl compound | Reaction conditionsa | 1,2,4-Trioxane 354 | Yield, % | Reference |
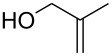 |
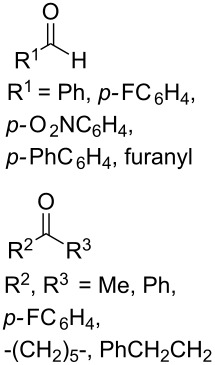 |
1) Co(acac)2, Et3SiH, O2, rt 2) TsOH |
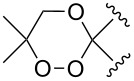 |
1) 60 2) 40–90 |
[403] |
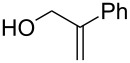 |
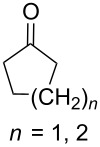 |
1) Co(acac)2, Et3SiH, O2, rt 2) TsOH |
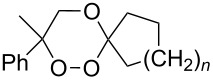 |
42 54 |
[403] |
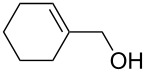 |
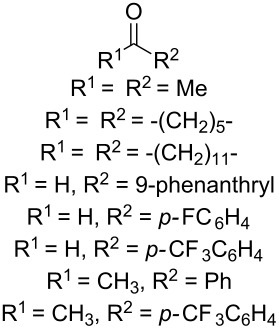 |
1) Co(thd)2, Et3SiH, O2, rt 2) TsOH |
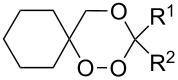 |
40–85 | [404] |
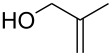 |
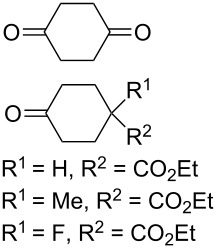 |
1) Co(acac)2, Et3SiH, O2, EtOH, 4 h 2) TsOH, CHCl3, 45 min to 3.5 h |
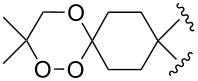 |
1) 36 2) 57–100 |
[153–158] |
a(Co(II)(thd)2) is bis(2,2,6,6-tetramethyl-3,5-heptanedionato)cobalt (II).
