Table 23.
Examples of 1,2,4-trioxanes 357 synthesized based on epoxides 355.
| Epoxide 355 | Carbonyl compound | Reaction conditions | 1,2,4-Trioxane 357 | Yield i) 356 ii) 357, % |
Ref. |
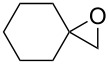 |
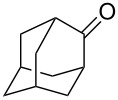 |
1) MoO2(acac)2, H2O2 , Et2O, MgSO4 2) TsOH, CH2Cl2, rt |
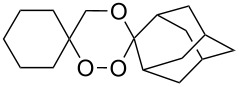 |
i) 59 ii) 95 |
[405] |
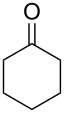 |
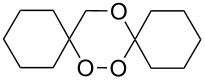 |
i) 59 ii) 69 |
[405] | ||
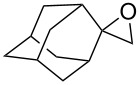 |
 |
1) MoO2(acac)2, H2O2, THF, MgSO4 2) 10-camphor- sulfonic acid, CH2Cl2, rt |
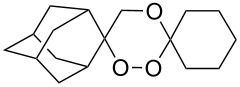 |
i) 29 ii) 46 |
[405] |
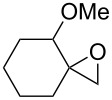 |
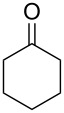 |
1) H2O2, Et2O, 0 °C, 4 h 2) H2SO4, CH2Cl2, rt, 4 d |
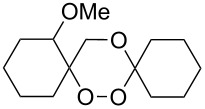 |
i) 8 ii) 28 |
[406] |
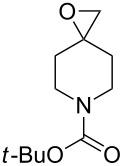 |
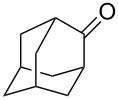 |
1) MoO2(acac)2, H2O2, Et2O, MgSO4, rt, 22 h 2) TsOH, CH2Cl2, rt, 5 h |
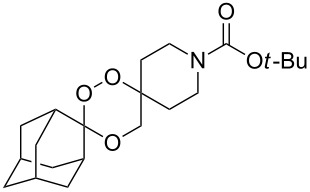 |
i) 98 ii) 92 |
[407] |
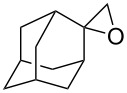 |
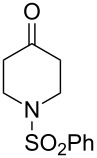 |
1) MoO2(acac)2, H2O2, Et2O 2) 10-camphor- sulfonic acid, CH2Cl2 |
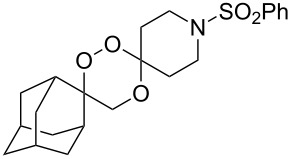 |
i) 25 ii) 39 |
[407] |
 |
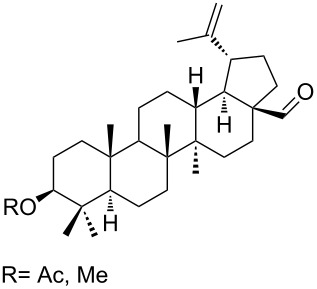 |
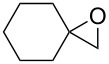
1) MoO2(acac)2, H2O2 , Et2O, MgSO4 2) BF3·Et2O, CH2Cl2, −78 °C to 0 °C, 5 h |
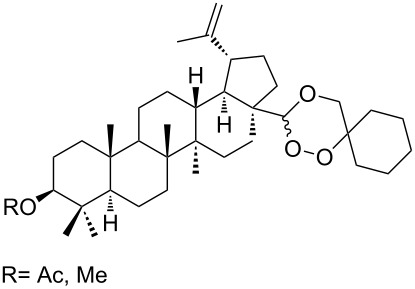 |
i) - ii) 27–35 |
[175] [176] |
