Fig. 5a–c.
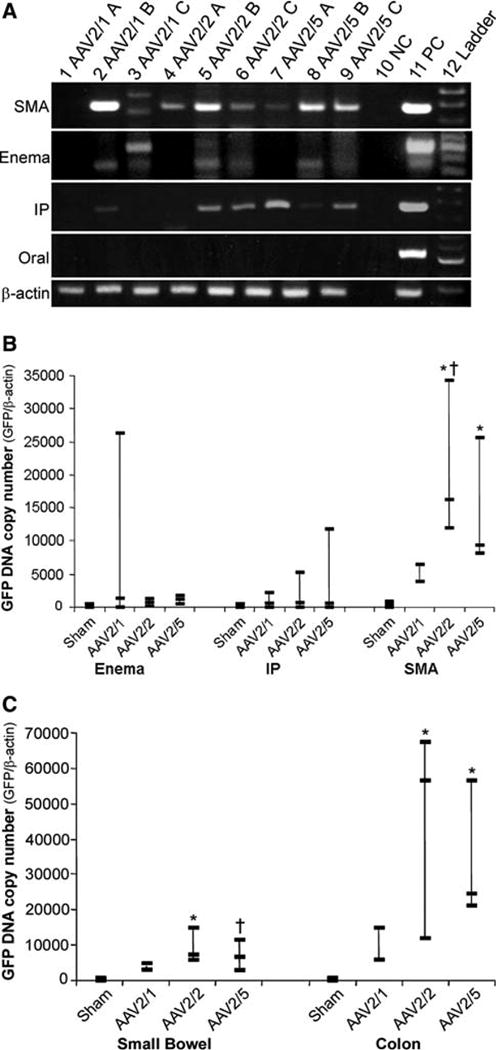
Detection and quantification of vector genomes. Tissue from animal treatment groups was initially screened for the presence of the GFP transgene by PCR. Pooled DNA extracted from random sections of both small intestine and colon was amplified for GFP by standard PCR. Products were examined on a 2% agarose gel where a GFP band (452 bp) could be visualized (a). Controls were run with each PCR experiment using DNA spiked with GFP DNA (PC) and DNA from sham-treated animals (NC). Quality of DNA used for PCR was checked by PCR amplification of mouse beta-actin (246 bp). Water was used in the beta-actin negative control PCR reaction. Gene transfer efficiency was compared between the three most positive treatment groups by determining GFP DNA copy number from pooled small intestine and colon DNA for each animal (b). SMA injection demonstrated better gene transfer efficiency as compared to other routes of administration. AAV2/2 showed higher copy numbers as compared to sham (*P < 0.005) and AAV2/1 (†P < 0.01) (b). AAV2/5 also revealed higher copy number compared to sham (*P = 0.005) (b). Further analysis of the SMA treatment group revealed a greater degree of gene transfer to the colon compared to the small intestine (c). AAV2/2 and 2/5 transduced the colon and the small bowel effectively as compared to sham (*P < 0.008, †P < 0.05) (c). Three animals were used per vector in each treatment group except the SMA AAV2/1 group which had only two animals
