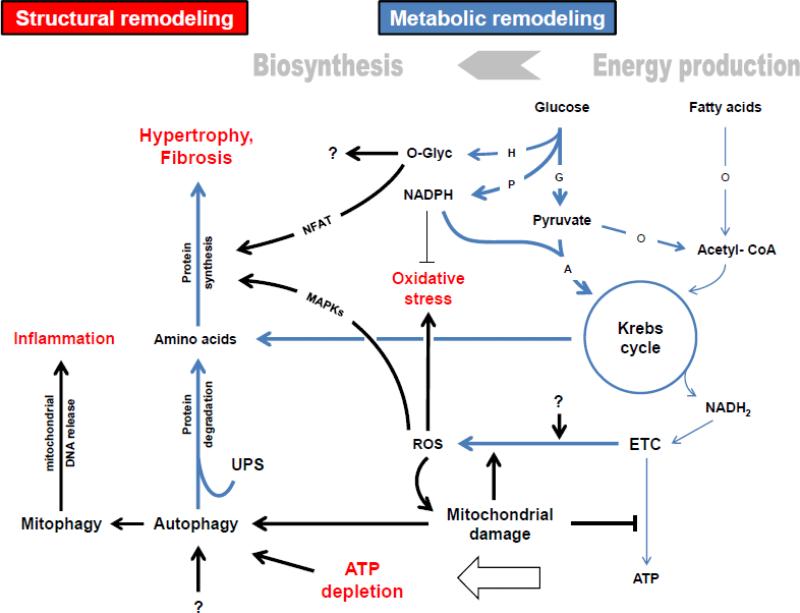
Figure 3. Overview of metabolic remodeling and proposed mechanisms linking it to other processes in the progression to HF.
H: Hexosamine biosynthetic pathway (HBP); P: Pentose phosphate pathway (PPP); G: Glycolysis; A: Anaplerosis; O: Oxidation; ETC: Electron transport chain; ROS: reactive oxygen species; UPS: ubiquitin-proteasome system. Metabolic pathways are blue. Bold lines indicate pathways/ processes that are increased or dominant. Thin lines represent pathways/ processes that are decreased. The question marks imply unknown causes/ effects. In general, metabolic remodeling in cardiac hypertrophy and failure is characterized by a shift away from energy production to activation of biosynthetic pathways required for structural remodeling processes such as ventricular hypertrophy and fibrosis. Particularly, fatty acid oxidation is decreased and may not be sufficiently compensated given the lack of increase in glucose oxidation. These alterations and further mitochondrial defects result in ATP depletion. Instead of being oxidized, pyruvate may be preferentially used for anaplerosis to maintain Krebs cycle moieties, which might be increasingly channeled into protein synthesis. Hypertrophic mediators such as MAPKs and NFAT are activated as a result of increased mitochondrial ROS and flux through the HBP, respectively. Overproduction of mitochondrial ROS causes oxidative damage. Although the flux through the PPP is increased, anti-oxidative defense might be inadequate due to the consumption of NADPH by the anaplerotic malic enzyme. Mitochondrial damage and ATP depletion may stimulate autophagy. Increased activity of autophagy and the UPS may contribute to hypertrophy by providing amino acids and other metabolites. Increase in mitophagy may trigger myocardial inflammation by releasing mitochondrial DNA.