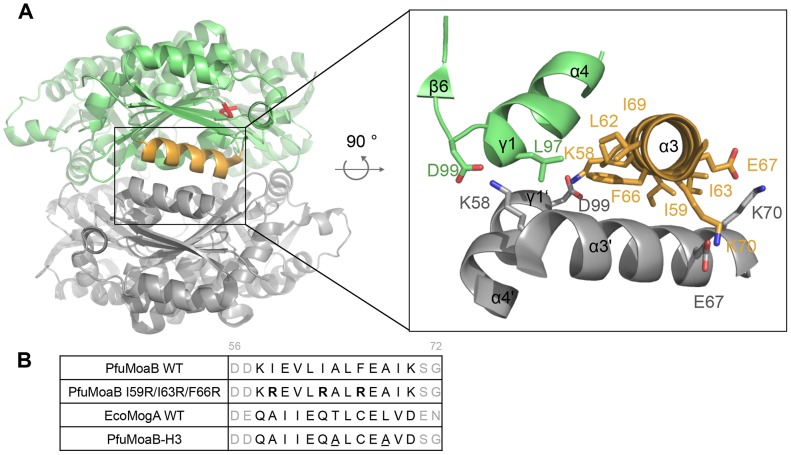
Figure 3. Hexamerization interface and structure-guided mutagenesis of PfuMoaB.
(A) Side view of PfuMoaB hexamer. Two trimers of PfuMoaB are depicted in grey and green. The conserved Gly-Gly-Thr-Gly motif within the active site is shown in the upper PfuMoaB monomer in red. The trimer-trimer interaction interface is boxed-in. α3-helix of the upper monomer is depicted in orange. The zoom-in shows labelled residues at the interface in stick representation. As the interface is build up by identical surfaces of each subunit, the hydrophobic residues of the bottom subunit (Ile59, Leu62, Ile63, Phe66, Ile69, Leu97) are not shown for the sake of clarity. (B) Structure-guided mutagenesis of PfuMoaB. Sequences of α3-helix of PfuMoaB-WT and EcoMogA are coloured in black, flanking residues of the helices are represented in grey. Residues are numbered accordingly to the PfuMoaB sequence. Sequences of the variants PfuMoaB I59R/I63R/F66R and PfuMoaB-H3 are shown. Arginine residues of PfuMoaB I59R/I63R/F66R are depicted in bold. Residues of PfuMoaB-H3, which were not exchanged accordingly to the EcoMogA sequence, are underlined.