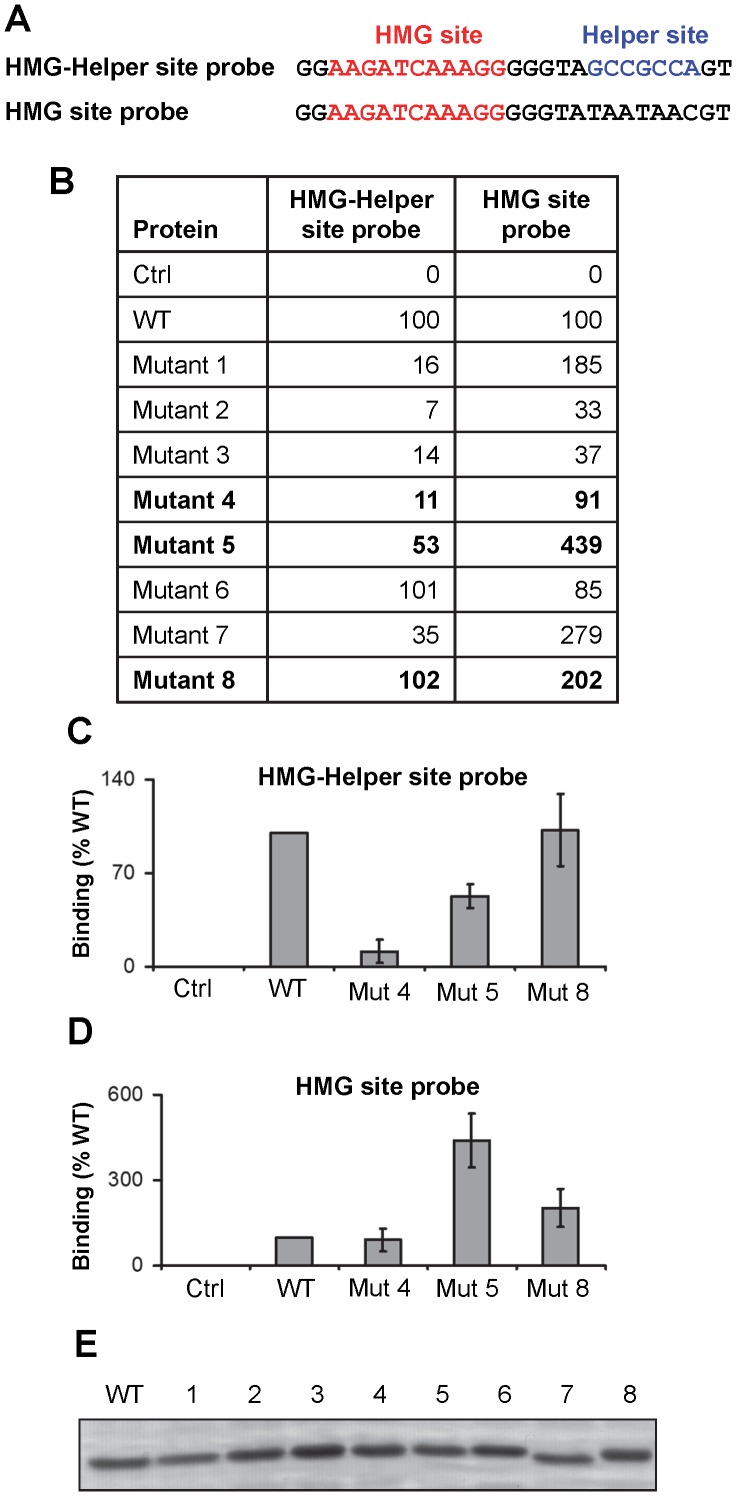
Figure 4. Characterization of the DNA-binding and inhibitory functions of the C-clamp.
(A) Sequence of the HMG-Helper site probe and the HMG site oligonucleotide probes used to characterize the ability of C-clamp mutants to bind DNA. (B) Protein fragments containing the HMG domain and wild type (WT) or mutant C-clamps were tested for their ability to bind the HMG-Helper site and the HMG site probes using the Licor EMSA assay described in Figure 1. Ctrl indicates probe only lane. For both probes, WT bound signal was normalized to 100. All mutants were tested at least twice and the averages are reported. Mutants 4, 5 and 8 are in bold, denoting their use in followup experiments. (C, D) EMSA experiments characterizing the defects in Mutants 4, 5 and 8 in binding to the HMG-Helper site (C) and HMG site (D) probes. For each binding reaction, 20 fmol of DNA probe and 9 pmol of protein was used. At these conditions, wild-type protein bound 7–12 times as much HMG-Helper site probe as HMG site probe (data not shown). Data represents means of triplicates±SD. These experiments were repeated three times with similar results. (E) Commassie stained gel of purified WT and C-clamp mutant proteins, demonstrating that each preparation used contained similar amounts of TCF/Pan.