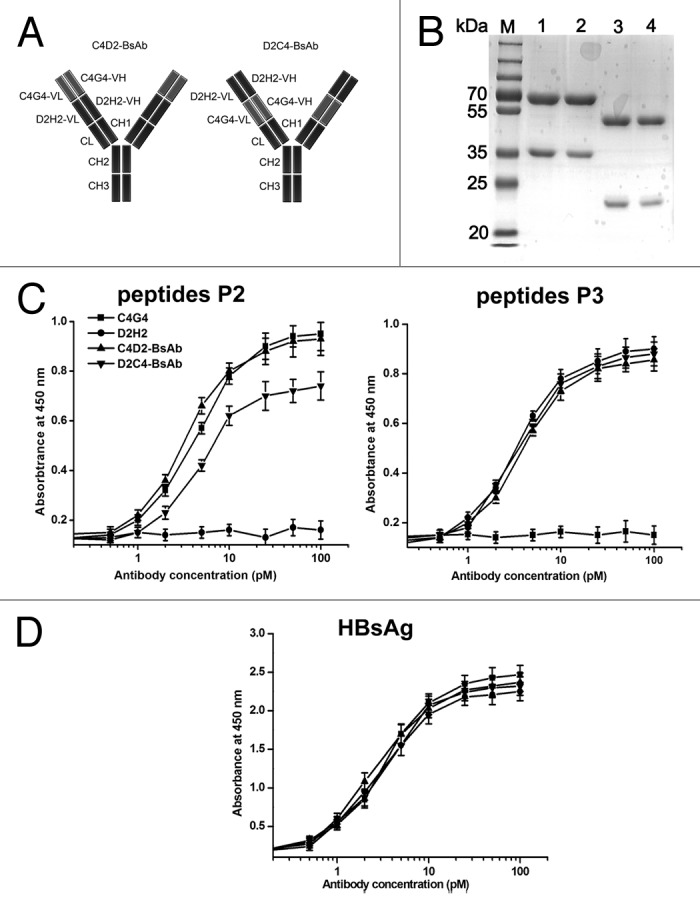
Figure 4. Schematic representation and characterization of bispecific antibodies. (A) Schematic representation of the structures of C4D2-BsAb and D2C4-BsAb. (B) SDS-PAGE analysis of purified antibodies under reducing conditions. Lane M, protein markers; lane 1, C4D2-BsAb; lane 2, D2C4-BsAb; lane 3, C4G4; lane 4, D2H2. (C and D) HBsAg-binding activity of bispecific antibodies. Increasing concentrations of C4G4, D2H2, C4D2-BsAb and D2C4-BsAb were added to 96-well plates coated with HBsAg or synthesized peptides P2 or P3. After 2 h incubation, the plates were washed and bound antibodies were detected with HRP-conjugated goat anti-human IgG. Results were shown as mean ± SD of three independent experiments.
