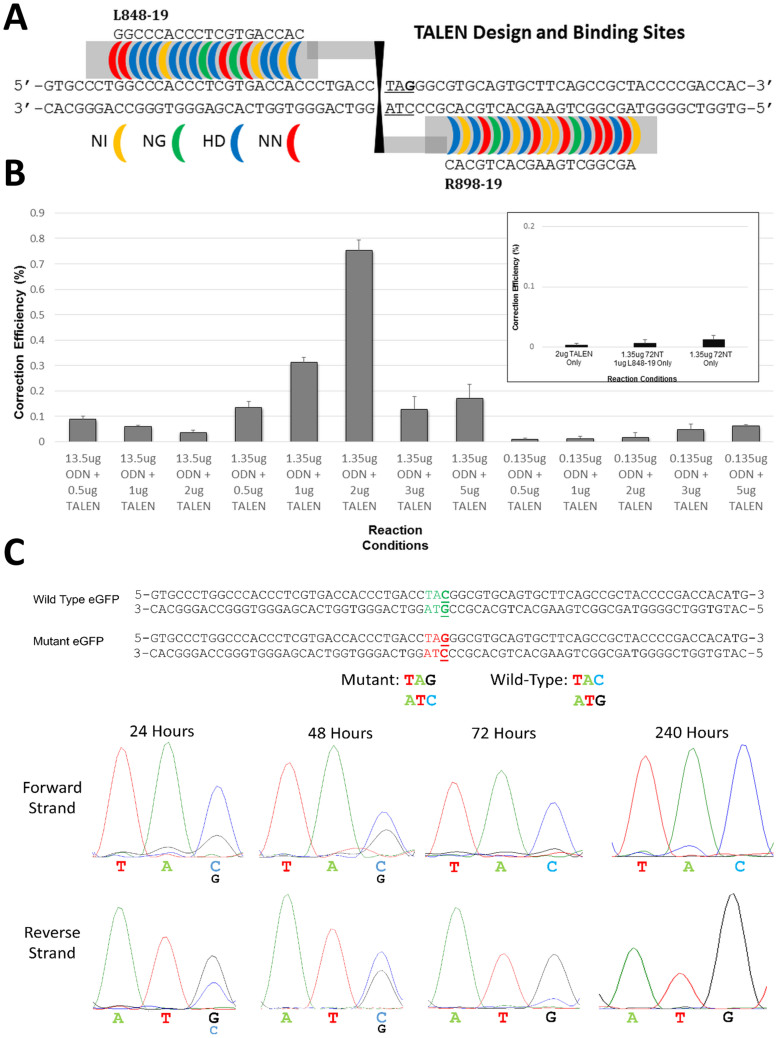
Figure 3. TALEN design and Gene Editing using TALENs and ssODNs.
(A). The TALEN pair, designed and built using the Golden Gate method, induces a double stranded break immediately preceding the mutant codon. RVDs are shown as color coded binding blocks next to their respective base, yellow NI:A, green NG:T, blue HD:C and red NN:G. Fok1 domains are shown in black and are positioned at their predicted cut site. (B). Unsynchronized HCT116-19 cells were harvested electroporated at a concentration of 5e5 cells/100 ul with TALENs and/or 72NT ODN at the indicated amounts. TALEN amounts reflect the total TALEN plasmid added to each sample in equal portions (1 ug L848-19 and 1 ug R898-19). Left and Right TALENs must be present for a DSB to be made. Following electroporation, cells were placed in 6-well plates and allowed to recover for 48 hours. Analyses took place on a Guava EasyCyte 5 HT flow cytometer (see Materials and Methods). Correction efficiency (%) was determined by the number of viable eGFP positive cells divided by the total viable cells in the population. Each treatment was performed in triplicate and error bars represent standard error. (C). Synchronized HCT116-19 cells were electroporated under the following conditions; 2 ug TALEN and 1.35 ug 72NT at 5e5 cells/100 ul. Cells were then sorted for GFP+ at 24, 48, 72 and 240 hours post electroporation. Immediately following cell sorting, DNA was isolated and the region surrounding the target base was amplified via PCR. Samples were submitted to Genewiz (South Plainfield, NJ) for sequencing analysis.