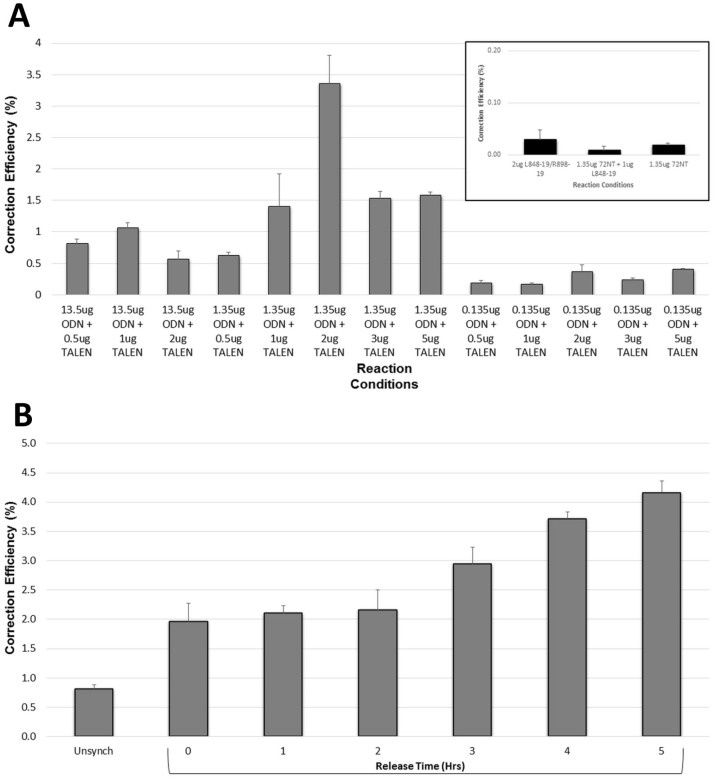
Figure 4. Gene editing of synchronized and released HCT116-19 cells using TALENs and ssODNs.
HCT116-19 cells were seeded at 2.5e6 cells in a 100 mm dish and synchronized for 24 hours with 6 uM aphidicolin then released for 4 hours before being electroporated at a concentration of 5e5 cells/100 ul with TALENs and/or the 72NT ODN at the amount indicted. TALEN amounts reflect the total TALEN plasmid added to each sample in equal portions (1 ug L848-19 and 1 ug R898-19). Left and Right TALENs must be present for a DSB to be made. Following electroporation, cells were seeded in 6-well plates and allowed to recover for 48 hours and analyses took place on a Guava EasyCyte 5 HT flow cytometer (see Materials and Methods). Correction efficiency (%) was determined by the number of viable eGFP positive cells divided by the total viable cells in the population. Each sample set was performed in triplicate and error bars represent standard error. (B). HCT116-19 cells were synchronized at the G1/S border with 6 uM aphidicolin for 24 hours; 5e6 cells/100 ul were then electroporated with a total of 2 ug TALEN at equal levels and 1.35 ug 72NT at 0, 1, 2, 3, 4 and 5 hours respectively, after release from aphidicolin. Following a 48 hr recovery, cells were analyzed for correction efficiency (%) via flow cytometry. Correction efficiency (%) was determined by the number of viable eGFP positive cells divided by the total viable cells in the population. For comparison, a population of unsynchronized cells treated similarly is also shown.