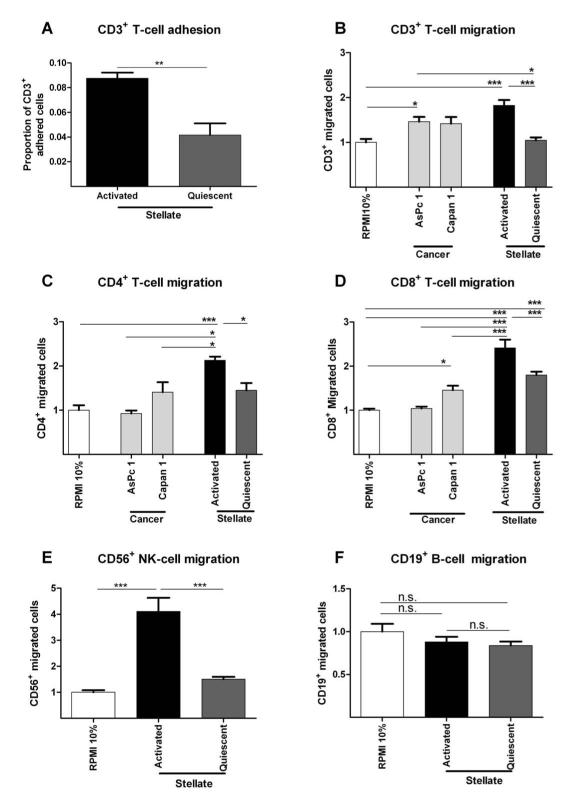
Figure 5. In vitro immune-stellate cells interactions.
Immuno-magnetic bead separated immune cells from un-diseased human donors (n=4) were used. Pancreatic stellate cells (PSC) were rendered quiescent using ATRA.11 Proportion of adherent T-cells to aPSCs or qPSCs were quantified after incubation for one hour and fixation and staining. (A; paired t-test; p-value is two-tailed). Transwell (5 μm) migration assays of different immune cells towards conditioned media (CM) from activated (aPSC), quiescent (qPSC) PSC and cancer cells (Capan1 and AsPc1) were carried out over 4-8 hours. Background migration of T-cells towards serum-free RPMI was subtracted to give ‘specific’ migration and normalized to migration towards RPMI supplemented with 10% FBS (‘basal’ serum directed migration) as shown in Supplementary Figure 13. These conditions served as internal controls for comparison across biological replicates.
We demonstrated reduced migration of CD3+ (B), CD4+ (C) and CD8+ (D), CD56+ (E) but not CD19+ qPSC CM compared with aPSC CM. The absolute CD4+ (B) cell number migration was minimal with little difference over the basal migration. Migration of all subsets of T-cells towards aPSC CM was higher than migration towards cancer cell CM. The most dramatic, and perhaps clinically relevant, fold change in migration of T-cells was seen with CD8+ T-cells (D) and NK cells (E).
Bar chart represents mean ± SEM. *** p< 0.001; ** p= 0.001 to 0.01; * p= 0.01 to 0.05, Comparisons were conducted with ANOVA with comparisons between columns using Bonferroni’s Multiple Comparison Test.