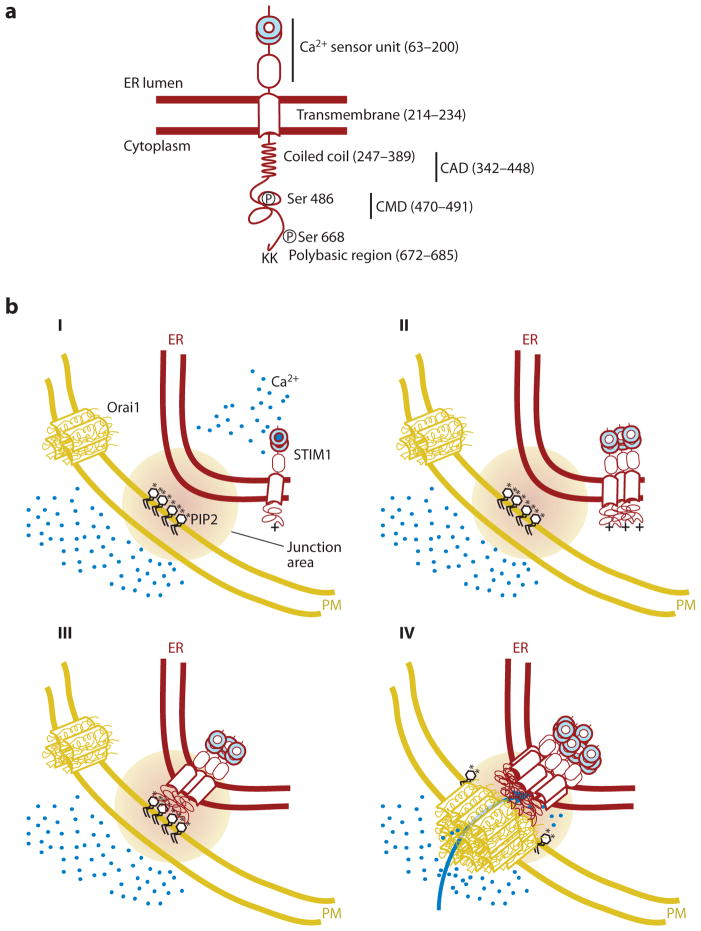
Figure 1.
Schematic representation of the STIM1 translocation process to endoplasmic reticulum (ER)–plasma membrane (PM) junctions following a decrease in ER Ca2+ levels. (a) Main regulatory domains of STIM1. Numbers in parenthesis depict domain-boundary residues in human STIM1. (b) Induced coupling of STIM1 and Orai1 at the ER-PM junction: (I) At high basal Ca2+ (blue circles) in the ER, STIM1 is present as a monomer or dimer relatively uniformly distributed in the ER. Orai1, likely a tetramer, is uniformly present in the PM and is inactive when not bound to STIM1. (II and III) When Ca2+ is depleted in the ER, Ca2+ dissociates from the STIM1 EF-SAM domain, changes its conformation, thereby inducing protein oligomerization, generating a bigger cluster of positively charged amino acids (plus sign), which is now sufficient to interact with PIP2 (black; phosphate group represented as an asterisk) and other negatively charged lipids in the PM. (IV) STIM1 localization to the ER-PM junction, together with an oligomerization-mediated conformational change of STIM1, triggers binding and activation of Orai1, further triggering Ca2+ entry across the PM. CAD, CRAC activation domain; CMD, CRAC modulatory domain.